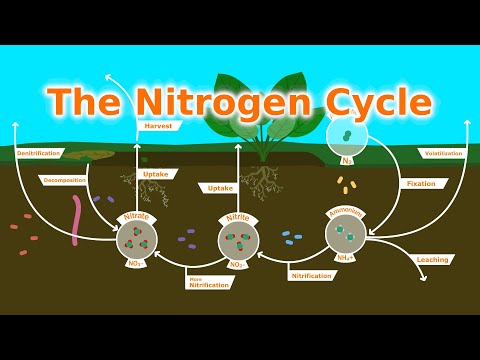
Q. Can animals get nitrogen from the air?
Animals and plants need nitrogen to build amino acids in proteins, which are the building blocks of life. Unlike oxygen, nitrogen cannot be absorbed directly from the air by animals and plants.
Q. Do most organisms use nitrogen directly from the air?
All living things need nitrogen to build proteins and other important body chemicals. However, most organisms, including plants, animals and fungi, cannot get the nitrogen they need from the atmospheric supply. They can use only the nitrogen that is already in compound form.
Table of Contents
- Q. Can animals get nitrogen from the air?
- Q. Do most organisms use nitrogen directly from the air?
- Q. What organisms can use atmospheric nitrogen?
- Q. Why can’t most organisms use the nitrogen in the air?
- Q. Which example is the biggest reservoir of nitrogen?
- Q. What are the four main reservoirs of nitrogen?
- Q. What is the largest source of nitrogen on Earth?
- Q. What are 2 ways nitrogen can be fixed?
- Q. What are three ways nitrogen can be fixed?
- Q. How do you remove nitrogen from the air in your home?
- Q. Is nitrogen a flammable gas?
- Q. What are the dangers of liquid nitrogen?
- Q. Is nitrogen a harmful gas?
- Q. How does a nitrogen leak kill you?
- Q. What is the most interesting fact about nitrogen?
- Q. What does nitrogen do to humans?
- Q. What are 5 interesting facts about nitrogen?
- Q. Why do we need nitrogen?
- Q. Is liquid nitrogen cold or hot?
- Q. What Colour is nitrogen?
Q. What organisms can use atmospheric nitrogen?
Cyanobacteria provide nitrogen to fungi by fixing atmospheric nitrogen. Rhizobium species are heterotrophic organisms growing in the roots of leguminous plants. Rhizobiums fix atmospheric nitrogen under low-oxygen pressure, and provide ammonium to plants.
Q. Why can’t most organisms use the nitrogen in the air?
Even though nitrogen gas makes up most of Earth’s atmosphere, plants cannot use this nitrogen gas to make organic compounds for themselves and other organisms. The two nitrogen atoms in a molecule of nitrogen gas are held together by a very stable triple bond. This bond must be broken for the nitrogen to be used.
Q. Which example is the biggest reservoir of nitrogen?
By far the largest reservoir of total nitrogen on Earth is the dinitrogen gas (N2) in the atmosphere (Table 4.1). N2 is also the major form of nitrogen in the ocean.
Q. What are the four main reservoirs of nitrogen?
Nitrogen moves slowly through the cycle and is stored in reservoirs such as the atmosphere, living organisms, soils, and oceans along its way. Most of the nitrogen on Earth is in the atmosphere.
Q. What is the largest source of nitrogen on Earth?
The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth’s atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen.
Q. What are 2 ways nitrogen can be fixed?
Nitrogen fixation in nature Nitrogen is fixed, or combined, in nature as nitric oxide by lightning and ultraviolet rays, but more significant amounts of nitrogen are fixed as ammonia, nitrites, and nitrates by soil microorganisms. More than 90 percent of all nitrogen fixation is effected by them.
Q. What are three ways nitrogen can be fixed?
Nitrogen fixation is the process by which nitrogen gas from the atmosphere is converted into different compounds that can be used by plants and animals. There are three major ways in which this happens: first, by lightning; second, by industrial methods; finally, by bacteria living in the soil.
Q. How do you remove nitrogen from the air in your home?
There are three standard methods used to extract nitrogen from air listed below: Cryogenic distillation. Pressure swing adsorption. Membrane nitrogen generation….Membrane nitrogen generation components include:
- Feed filter coalescers.
- Immersion heaters.
- Activated carbon filters.
- Particulate filters.
Q. Is nitrogen a flammable gas?
EMERGENCY OVERVIEW: Nitrogen is a colorless, odorless, non-flammable gas, or a colorless, odorless, cryogenic liquid. The main health hazard associated with releases of this gas is asphyxiation, by displacement of oxygen.
Q. What are the dangers of liquid nitrogen?
Liquid nitrogen has a boiling temperature of -196°C at atmospheric pressure. Direct contact can freeze the skin causing frostbite and cold burns. Delicate tissue, such as eyes, can be damaged by an exposure to the cold gas alone which would be too brief to affect skin.
Q. Is nitrogen a harmful gas?
High concentrations of nitrogen gas can be particularly harmful to human health. Nitrogen can displace oxygen from ambient air within an enclosed space leading to a dangerous build-up of the inert gas.
Q. How does a nitrogen leak kill you?
After just two or three breaths of nitrogen, the oxygen concentration in the lungs would be low enough for some oxygen already in the bloodstream to exchange back to the lungs and be eliminated by exhalation. Unconsciousness in cases of accidental asphyxia can occur within 1 minute.
Q. What is the most interesting fact about nitrogen?
Nitrogen is 75% of the air we breathe. All living things contain nitrogen, mostly in amino acids, DNA, and RNA. The human body contains about 3% nitrogen, making it the fourth most prevalent element after oxygen, carbon and hydrogen. Nitrogen is required to build amino acids.
Q. What does nitrogen do to humans?
Nitrogen is an essential element for all forms of life and is the structural component of amino acids from which animal and human tissues, enzymes, and many hormones are made.
Q. What are 5 interesting facts about nitrogen?
Facts:
- N has no odor, is tasteless, and colorless.
- Nitrogen gas (N2) makes up 78.1% of the Earth’s atmosphere.
- Atmosphere contains an estimated 4,000 trillion tons of N.
- Nitrogen is not a metal.
- Nitrogen gas is inert.
- French chemist Antoine Laurent Lavoisier named nitrogen azote, meaning without life.
Q. Why do we need nitrogen?
Nitrogen is an essential nutrient for the production of amino acids, proteins, nucleic acids, etc., and stone fruit trees require an adequate annual supply for proper growth and productivity. Nitrogen is primarily absorbed through fine roots as either ammonium or nitrate.
Q. Is liquid nitrogen cold or hot?
Liquid nitrogen is just very cold nitrogen. It is 320oF below zero (-196oC). It’s so cold that it freezes anything it touches almost instantly. Also, anything at normal room temperature is so much hotter than liquid nitrogen that the liquid nitrogen boils when it touches something.
Q. What Colour is nitrogen?
blue