When the reactants are mixed, the temperature change caused by the reaction is an indicator of a chemical change. This reaction generates heat as a product and is (very) exothermic. However, physical changes can be exothermic or endothermic. Thus, it is a physical change.
Q. How much energy is absorbed or released in nuclear reaction?
The amount of energy released during nuclear fission is millions of times more efficient per mass than that of coal considering only 0.1 percent of the original nuclei is converted to energy. Daughter nucleus, energy, and particles such as neutrons are released as a result of the reaction.
Table of Contents
Q. Does a physical change release a lot of energy?
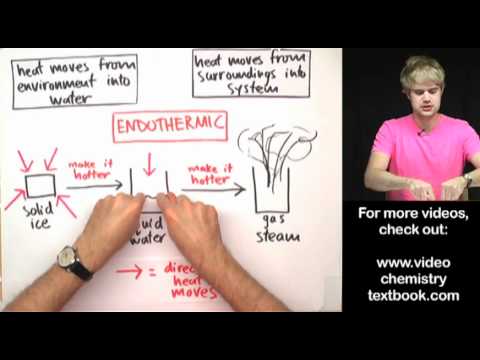
During a physical change in matter, such as the evaporation of liquid water to water vapour, the energy of the water molecules increases. However, the change in energy is much smaller than in chemical reactions. When a chemical reaction occurs, some bonds will break, while new bonds may form.
Q. Is heating a pan exothermic or endothermic?
Solution: endothermic – you must put a pan of water on the stove and give it heat in order to get water to boil. Because you are adding heat/energy, the reaction is endothermic. exothermic – when you burn something, it feels hot to you because it is giving off heat into the surroundings.
Q. Is breaking down food endothermic or exothermic?
exothermic reaction. The digestion of food is Endothermic Reaction because Energy is formed during the process. digestion of food is endothermic reaction because heat or energy is utilized to break down of food ..
Q. Is lighting a match an exothermic reaction?
Striking A Match. When a match is lit, potassium, chlorine, phosphorus, and sulfur react and cause a combustion, which produces light and heat. This chemical reaction is exergonic because it releases energy and exothermic because it releases heat.
Q. Is burning a match exo or endothermic?
A match requires initial energy, provided by the heat generated from the friction as it strikes the rough surface on the matchbox to ignite it. Once the match starts burning, it releases more energy than was required for ignition so the reaction is still exothermic.