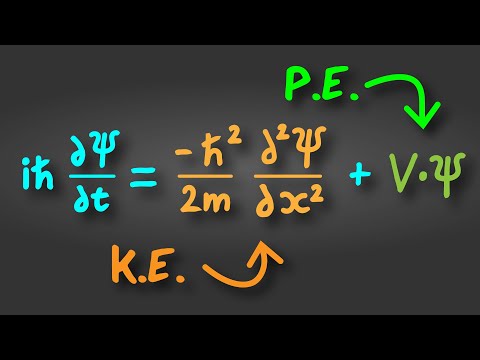Q. Can we prove Schrodinger equation?
In the new study, the scientists have shown that it’s possible to obtain the Schrödinger equation from a simple mathematical identity, and found that the mathematics involved may help answer some of the fundamental questions regarding this important equation.
Q. What is K in Schrodinger equation?
Curvature of Wave Functions The simplest example is that of a constant potential V(x)=V0kx+δ) with δ a constant and k=√(2m/ℏ2)(E−V0). Only with exactly the right initial conditions will the curvature be just right to bring the wave function to zero as x goes to infinity.
Table of Contents
- Q. Can we prove Schrodinger equation?
- Q. What is K in Schrodinger equation?
- Q. How do you pronounce Schrodinger?
- Q. How is Ö pronounced?
- Q. What is time independent Schrodinger equation?
- Q. Is Schrödinger German?
- Q. What did Schrodinger’s Cat experiment prove?
- Q. What was Schrodinger’s discovery?
- Q. Is the cat alive or dead?
- Q. What are Bohr’s 4 postulates?
- Q. What is Z in Bohr’s equation?
- Q. What was Rutherford’s model called?
- Q. Why did Bohr’s model fail?
- Q. Why does the Bohr model not work for helium?
- Q. Why is Rutherford’s model called the peach?
- Q. What was Rutherford’s experiment?
- Q. How did JJ Thomson discovered the electron?
- Q. Who named Proton?
- Q. Who is the father of neutron?
- Q. Who is the father of electron?
- Q. What did E Goldstein discover?
Q. How do you pronounce Schrodinger?
The correct pronunciation of Erwin Schrödinger is EHR-veen SHROE-deeng-uhr.
Q. How is Ö pronounced?
To pronounce the ö-sound, say “ay” as in day (or as in the German word See). While continuing to make this sound, tightly round your lips. Look in a mirror to make sure your lips are actually rounded. Voilà!
Q. What is time independent Schrodinger equation?
The time independent Schrodinger equation for one dimension is of the form. where U(x) is the potential energy and E represents the system energy. It has a number of important physical applications in quantum mechanics.
Q. Is Schrödinger German?
Erwin Rudolf Josef Alexander Schrödinger (UK: /ˈʃrɜːdɪŋər/, US: /ˈʃroʊ-/; German: [ˈɛɐ̯viːn ˈʃʁøːdɪŋɐ]; 12 August 1887 – 4 January 1961), sometimes written as Erwin Schrodinger or Erwin Schroedinger (“oe” is the proper transliteration of the German “ö”), was a Nobel Prize-winning Austrian-Irish physicist who developed …
Q. What did Schrodinger’s Cat experiment prove?
“Schrodinger’s Cat” was not a real experiment and therefore did not scientifically prove anything. Schrodinger constructed his imaginary experiment with the cat to demonstrate that simple misinterpretations of quantum theory can lead to absurd results which do not match the real world.
Q. What was Schrodinger’s discovery?
Erwin Schrödinger showed that the quantization of the hydrogen atom’s energy levels that appeared in Niels Bohr’s atomic model could be calculated from the Schrödinger equation, which describes how the wave function of a quantum mechanical system (in this case, a hydrogen atom’s electron) evolves.
Q. Is the cat alive or dead?
Many-worlds interpretation and consistent histories The cat is both alive and dead—regardless of whether the box is opened—but the “alive” and “dead” cats are in different branches of the universe that are equally real but cannot interact with each other.
Q. What are Bohr’s 4 postulates?
Bohr’s model of the hydrogen atom is based on three postulates: (1) an electron moves around the nucleus in a circular orbit, (2) an electron’s angular momentum in the orbit is quantized, and (3) the change in an electron’s energy as it makes a quantum jump from one orbit to another is always accompanied by the …
Q. What is Z in Bohr’s equation?
Bohr’s model allows classical behavior of an electron (orbiting the nucleus at discrete distances from the nucleus. The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element.
Q. What was Rutherford’s model called?
the nuclear model
Q. Why did Bohr’s model fail?
The Bohr model failed because it failed to take into account one thing: synchrotron radiation. The Bohr model says that electrons whizz around the atomic nucleus in the same way that the earth goes around the sun – but rather than being bound by gravity, it is bound by electromagnetic forces.
Q. Why does the Bohr model not work for helium?
Bohr’s theory had major drawbacks, however. Except for the spectra of X-rays in the K and L series, it could not explain properties of atoms having more than one electron. The binding energy of the helium atom, which has two electrons, was not understood until the development of quantum mechanics.
Q. Why is Rutherford’s model called the peach?
Rutherford’s model of the atom was nicknamed the peach because his depiction of the atom’s structure showed a dense core at the center of the atom…
Q. What was Rutherford’s experiment?
Ernest Rutherford’s most famous experiment is the gold foil experiment. A beam of alpha particles was aimed at a piece of gold foil. Most alpha particles passed through the foil, but a few were scattered backward. This showed that most of the atom is empty space surrounding a tiny nucleus.
Q. How did JJ Thomson discovered the electron?
In 1897, J.J. Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube. Thomson realized that the accepted model of an atom did not account for negatively or positively charged particles. Therefore, he proposed a model of the atom which he likened to plum pudding.
Q. Who named Proton?
Ernest Rutherford
Q. Who is the father of neutron?
Sir James Chadwick
Q. Who is the father of electron?
Sir Joseph John Thomson OM
Q. What did E Goldstein discover?
He was primarily interested in electrical discharges in moderate to high vacuums. In 1886 he discovered what he termed Kanalstrahlen, or canal rays, also called positive rays; these are positively charged ions that are accelerated toward and through a perforated cathode in an evacuated tube.