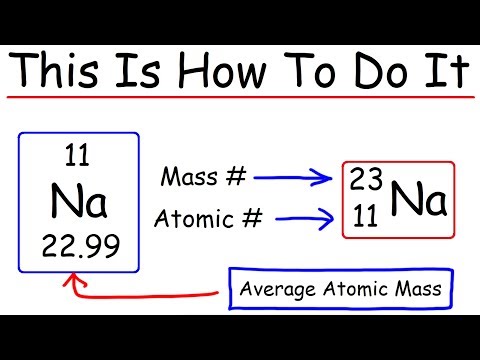
Q. Do atoms have the same number of protons and neutrons?
Atoms of a particular element must have the same number of protons but can have different numbers of neutrons. When an element has different variants that, while all having the same number of protons, have differing numbers of neutrons, these variants are called isotopes.
Q. Who invented isobars?
The term “isobars” (originally “isobares”) for nuclides was suggested by Alfred Walter Stewart in 1918.
Table of Contents
Q. Why do isobars exist?
Atoms of chemical elements having same atomic mass but a different atomic number are called Isobars. The sum of the number of protons and neutrons together form the atomic mass. Therefore, they are always different chemical elements having same atomic masses. Thus, isobar has different chemical properties.
Q. How many isobars are there?
Isobar, in nuclear physics, any member of a group of atomic or nuclear species all of which have the same mass number—that is, the same total number of protons and neutrons. Thus, chlorine-37 and argon-37 are isobars.
Q. Which pairs are isobars?
isobars differ in atomic number (or number of protons) but have the same mass number. Anexample of a series of isobars would be 40S, 40Cl, 40Ar, 40K, and 40Ca. The nuclei of these nuclides all contain 40 nucleons; however, they contain varying numbers of protons and neutrons.
Q. What is an Isobar?
Isobar, line on a weather map of constant barometric pressure drawn on a given reference surface. The isobaric pattern on a constant-height surface is extremely useful in weather forecasting because of the close association between pressure and weather.
Q. What causes isotopes to form?
Isotopes can either form spontaneously (naturally) through radioactive decay of a nucleus (i.e., emission of energy in the form of alpha particles, beta particles, neutrons, and photons) or artificially by bombarding a stable nucleus with charged particles via accelerators or neutrons in a nuclear reactors.