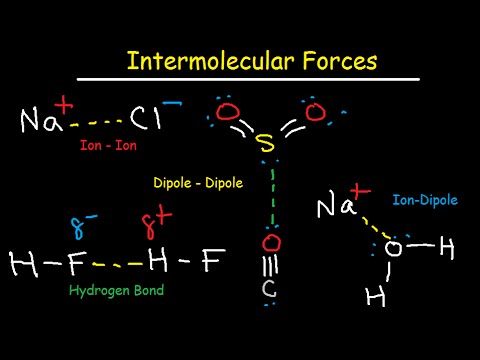
Ionic compounds have ionic forces. Covalent compounds all have London dispersion (LD) forces, whereas polar covalent compounds have dipole forces and/or hydrogen-bonding forces. For hydrogen bonding (H-bonding) forces, the covalent compound must have either a N−H, O−H, or F−H bond in the molecule.
Q. Does cacl2 have ion dipole forces?
An ion-dipole force requires ions (generally a soluble salt such as NaCl or CaCl2) and a polar solvent (like water or rubbing alcohol).
Table of Contents
Q. Does oil have dipole-dipole forces?
Oil is a non-polar molecule, while water is a polar molecule. When oil and water are mixed, the dipole-dipole interactions are disrupted, but constant molecular motion allows the stronger dipole-dipole attractions to partition the polar molecules from the mixture.
Q. What intermolecular forces does HF have?
Intermolecular Forces HF is a polar molecule: dipole-dipole forces. Hydrogen is bounded to F. Hydrogen bonds exist. There are also dispersion forces between HBr molecules.
Q. What forces does NaCl have?
Dipole-dipole forces are probably the simplest to understand. You probably already know that in an ionic solid like NaCl, the solid is held together by Coulomb attractions between the oppositely-charges ions. The Na+ and Cl- ions alternate so the Coulomb forces are attractive.
Q. What holds atoms together in HF?
…and fluorine react to form hydrogen fluoride, which contains HF molecules. The hydrogen and fluorine atoms are bound together by a pair of electrons, one electron contributed by the hydrogen atom and one by the fluorine atom. Although the electrons are shared between the hydrogen and the fluorine atoms, in…
Q. Which type of force exist in HF?
The intermolecular forces present between HF molecules are mainly dipole interaction and Hydrogen bonds.
Q. What is holding two h2o molecules together?
Strong linkages—called covalent bonds—hold together the hydrogen (white) and oxygen (red) atoms of individual H2O molecules. Covalent bonds occur when two atoms—in this case oxygen and hydrogen—share electrons with each other.