Electrons are positively charged and located in the nucleus together with the neutrons. Electrons have a charge of +1 and a mass of approximately 1 atomic mass unit (amu). Every element has a unique Element Symbol and a unique Atomic Number which can be accessed via the Periodic Table with Atomic Mass.
Q. Is the atomic number the number of electrons?
The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons. The sum of the atomic number Z and the number of neutrons N gives the mass number A of an atom.
Table of Contents
- Q. Is the atomic number the number of electrons?
- Q. Which element has the greatest number of electrons?
- Q. Which element has a unique number of?
- Q. Why does sodium have 12 neutrons?
- Q. What is a proton plus a neutron?
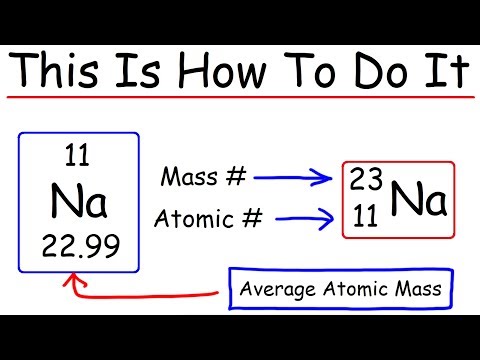
- Q. How many protons are there in an atom of 23 11 NA?
- Q. How many electrons does K have?
- Q. How many protons and electrons are in Na+?
- Q. How many neutrons are there in chlorine 37?
Q. Which element has the greatest number of electrons?
uranium element
Q. Which element has a unique number of?
Every atom of a particular element contains the same number of protons. Each element has a unique atomic number, or a unique number of protons in its nucleus.
Q. Why does sodium have 12 neutrons?
We know that the atomic number of sodium is 11. This tells us that sodium has 11 protons and because it is neutral it has 11 electrons. Since sodium has 11 protons, the number of neutrons must be 23 – 11 = 12 neutrons.
Q. What is a proton plus a neutron?
The mass number of an atom is equal to the number of protons plus the number of neutrons that it contains. In other words, the number of neutrons in any atom is its mass number minus its atomic number. Number of neutrons = mass number – atomic number.
Q. How many protons are there in an atom of 23 11 NA?
11 protons
Q. How many electrons does K have?
19 electrons
Q. How many protons and electrons are in Na+?
There are 10 electrons present in Na+. The atom of sodium has 11 electrons, 11 protons along with 12 neutrons, but Na+ contains one less electron, 11 protons along with 12 neutrons, as the ion has lost 1 electron.
Q. How many neutrons are there in chlorine 37?
20 neutrons