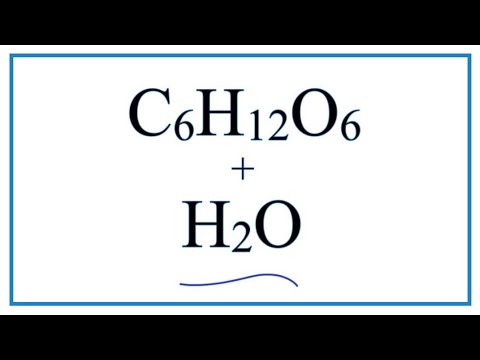
The reason glucose dissolves readily in water is because it has lots of polar hydroxyl groups which can hydrogen-bond with water molecules. Hydrogen bonds are very important intermolecular forces which determine the shape of molecules like DNA, proteins and cellulose.
Q. Why does glucose dissolve in water?
Glucose is small (6 carbons) and dissolves easily in water because it has a number of polar OH groups attached to its carbons. To these cells, glucose is fuel and raw material for making other biological molecules.
Table of Contents
- Q. Why does glucose dissolve in water?
- Q. What happens when glucose dissolves in water?
- Q. Why do they put calcium chloride in water?
- Q. What can I use instead of calcium chloride?
- Q. What happens when calcium chloride is exposed to air?
- Q. What are the benefits of calcium chloride?
- Q. Is calcium chloride positive or negative?
- Q. Is calcium chloride a chlorine?
- Q. Is calcium chloride in pool shock?
- Q. Can I swim after adding calcium chloride?
- Q. Can I use calcium chloride ice melt in my pool?
- Q. Can I use calcium chloride in my pool?
- Q. What is calcium chloride for the pool?
- Q. Does baking soda raise calcium hardness?
- Q. Does calcium chloride change pool pH?
Q. What happens when glucose dissolves in water?
The sugar we use to sweeten coffee or tea is a molecular solid, in which the individual molecules are held together by relatively weak intermolecular forces. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these C12H22O11 molecules are released into solution.
Q. Why do they put calcium chloride in water?
According to expert opinion, calcium chloride is safe to consume. It’s added to water for taste and serves as an electrolyte to keep you from getting dehydrated.
Q. What can I use instead of calcium chloride?
Calcium Chloride Alternatives
- Organic Oils. Organic oils such as vegetable oils, pine tar and molasses are an option because these substances stick to dust and dirt particles and keep them from escaping into the air.
- Positive Ionic Attraction.
- Enzyme Mixtures.
- Magnesium Chloride.
Q. What happens when calcium chloride is exposed to air?
If anhydrous calcium chloride is exposed in the air what it will generally happen is that it will absorb water from the atmosphere. Then after few minutes it will be observed that there will be pool of liquid which is remained back instead of a lump which is basically white in color.
Q. What are the benefits of calcium chloride?
Calcium Chloride is a mineral indicated in the immediate treatment of hypocalcemic tetany (abnormally low levels of calcium in the body that cause muscle spasm). Calcium chloride injection is also used in cardiac resuscitation, arrhythmias, hypermagnesemia, calcium channel blocker overdose, and beta-blocker overdose.
Q. Is calcium chloride positive or negative?
Since calcium lost two electrons, it has 20 protons, but only 18 electrons. This makes calcium a positive ion with a charge of 2+. Since each chlorine atom gained an electron, they each have 17 protons and 18 electrons. This makes each chloride a negative ion with a charge of −1.
Q. Is calcium chloride a chlorine?
Chloride: The negatively charged ionic form of Chlorine. Chloride is found abundantly in nature and is most commonly known for forming neutral salts such as sodium chloride (table salt), potassium chloride, and calcium chloride.
Q. Is calcium chloride in pool shock?
Calcium chloride doesn’t dissolve the same way as dry acid, sodium bicarb or a non-chlorine shock; calcium chloride gives off a lot of heat. Like any other dry chemical, however, calcium chloride should be pre-dissolved in a bucket prior to adding to the pool.
Q. Can I swim after adding calcium chloride?
After Adding Calcium Chloride to Raise Calcium Hardness: You should wait 2-4 hours (or one full cycle through the filter) to swim from the moment you use calcium chloride in your pool.
Q. Can I use calcium chloride ice melt in my pool?
No you cannot. The calcium chloride ice melter has more than 90% calcium chloride. Adding it to you pool will only increase the calcium hardness.
Q. Can I use calcium chloride in my pool?
Calcium chloride is so essential to your swimming pool because it contributes greatly to the structural health of the pool itself. Adding calcium chloride to your pool will increase the overall calcium hardness levels in your pool. This fortification is essential.
Q. What is calcium chloride for the pool?
Calcium chloride for pools is a chemical compound used to maintain pool health. Pool water can be ‘hard’ water, or ‘soft’ water, depending on the dissolved mineral content. Calcium chloride balances the water.
Q. Does baking soda raise calcium hardness?
In the case where too much baking soda is added to hard water, it can cause a build-up of calcium around your pool.
Q. Does calcium chloride change pool pH?
The pH of the pool water is maintained by adding sodium bicarbonate (See note on alkalinity) which increases the pH. Correcting the hardness by adding Calcium Chloride (see note on hardness) will also increase the pH value. If it does exceed 7.6 then the pH can be reduced by the addition of Sodium bisulphate.