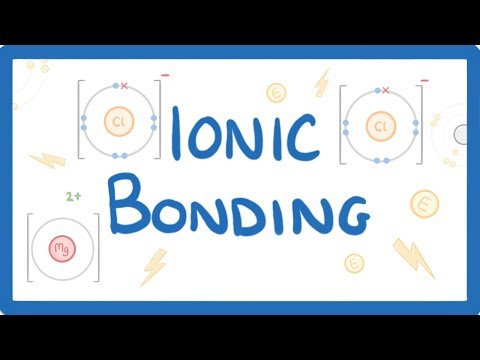
Q. Does sodium form ionic bonds?
An atom of sodium (Na) donates one of its electrons to an atom of chlorine (Cl) in a chemical reaction, and the resulting positive ion (Na+) and negative ion (Cl−) form a stable ionic compound (sodium chloride; common table salt) based on this ionic bond. …
Q. What type of bond is sodium?
Metallic bonding
Table of Contents
- Q. Does sodium form ionic bonds?
- Q. What type of bond is sodium?
- Q. Why does sodium form ionic bonds?
- Q. Is sodium ionic or covalent or metallic?
- Q. Which bond is strongest covalent or ionic?
- Q. What two factors influence the strength of covalent bonds?
- Q. Why is energy required to break a covalent bond?
- Q. How is a hydrogen bond different from an ionic or covalent bond?
- Q. What kind of bonds does the CC bond in C2H2 have?
- Q. How many double bonds does C2H2?
- Q. How many sigma and pi bonds does c2h2?
Q. Why does sodium form ionic bonds?
Because of the propensity of sodium to lose an electron and of chlorine to gain an electron, the elements are well suited to bond with one another. This transfer of electrons results in the formation of the ionic bond holding Na+ and Cl– together.
Q. Is sodium ionic or covalent or metallic?
Ionic bonds form when atoms transfer electrons between each other, forming ions that are electrically attracted to each other forming a bond between them. Sodium chloride (NaCl) is a typical ionic compound. The picture below shows both a sodium and a chlorine ion.
Q. Which bond is strongest covalent or ionic?
Ionic bond is much stronger than covalent bond because it involves complete transfer of electrons because of which there is formation of cation and anion and there exist huge electrostatic forces of attraction. They also have high melting and boiling point which proves that the ionic bond is very strong.
Q. What two factors influence the strength of covalent bonds?
The higher positive charge,small cation size and larger an ion the covalent bond result. In ionic bonds, charge and distance are the two factors that affect the strength of the bond.
Q. Why is energy required to break a covalent bond?
Energy is required to break bonds. Atoms are much happier when they are “married” and release energy because it is easier and more stable to be in a relationship (e.g., to generate octet electronic configurations). The enthalpy change is negative because the system is releasing energy when forming bond.
Q. How is a hydrogen bond different from an ionic or covalent bond?
unlike ionic or covalent bonds, in which electrons are given up or shared, the hydrogen bond is a weaker attraction. Hydrogen bond are generally intermolecular, while ionic and covalent bonds occur between ions or respectively. hydrogen bonding exists between water molecules, but not between hydrogen sulfide molecules.
Q. What kind of bonds does the CC bond in C2H2 have?
Ethyne, C2H2, has a triple bond between the two carbon atoms. In the diagram each line represents one pair of shared electrons.
Q. How many double bonds does C2H2?
The C2H2 molecule contains a triple bond between the two carbon atoms, one of which is a sigma bond, and two of which are pi bonds.
Q. How many sigma and pi bonds does c2h2?
It has 3 σ-bond and 2 π bond.