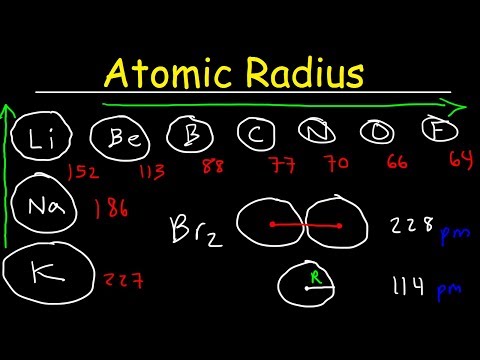
Q. Does TI or as have a larger atomic radius?
As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Thus, helium is the smallest element, and francium is the largest….Periodic Trends — Atomic and Ionic Radii.
| Sc 161 | |
| Ti 145 | |
| V 132 | |
| Cr 125 | |
| 4A | Ge 123 |
Q. Which has a bigger radius?
An atomic radius is one-half the distance between the nuclei of two atoms. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). Hydrogen (H) has the smallest average atomic radius at about 25 pm, while caesium (Cs) has the largest average radius at about 260 pm.
Table of Contents
- Q. Does TI or as have a larger atomic radius?
- Q. Which has a bigger radius?
- Q. Which of these atoms has the largest radius?
- Q. What is the atomic radius of Al?
- Q. Which cation has smallest radius?
- Q. Which ion has the largest radius?
- Q. Which cation has the smallest radius K Na Li?
- Q. What is the atomic radius of RB?
- Q. Which of the following has the smallest radius?
- Q. What is PM atomic radius?
- Q. Why is the atomic radius of k larger than Br?
- Q. Are there any exceptions to the atomic radius trend?
- Q. Does atomic radius increase from top to bottom?
- Q. Which is the best reason that the atomic radius generally increases?
- Q. Does atomic radius increase down a group?
- Q. How do you think the atomic radius will change as electrons are added to a shell?
- Q. How do you think the atomic radii will change as electrons are added to the shell?
- Q. Why does the atomic radius decrease as electrons are added to a shell quizlet?
- Q. Why does atomic radius increase from top to bottom in a chemical family quizlet?
- Q. Why does atomic size increase down a group and decrease left to right?
- Q. Why does atomic size increase from right to left?
- Q. Which has a larger atomic radius carbon or oxygen?
- Q. Which has highest electron affinity?
- Q. How do you find electron affinity?
- Q. Which has a larger atomic radius aluminum or oxygen?
- Q. Does fluorine or iodine have a larger atomic radius?
- Q. Is Carbon larger than oxygen?
Q. Which of these atoms has the largest radius?
Cesium
Q. What is the atomic radius of Al?
184 pm
Q. Which cation has smallest radius?
So, in the end, we can conclude that nickel ions have a minimum radius. So, the correct answer is Option C. Note: While comparing the ionic radius, we should consider all the points.
Q. Which ion has the largest radius?
K+
Q. Which cation has the smallest radius K Na Li?
(A) Comparison of Atomic and Ionic Radius of Group 1 (IA, alkali metals) Elements
| Element | Symbol of Atom | Trend |
|---|---|---|
| lithium | Li | smallest ↓ |
| sodium | Na | ↓ |
| potassium | K | ↓ |
| rubidium | Rb | ↓ |
Q. What is the atomic radius of RB?
290 pm
Q. Which of the following has the smallest radius?
Helium has the smallest atomic radius.
Q. What is PM atomic radius?
Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter), or between 0.3 and 3 ångströms. Therefore, the radius of an atom is more than 10,000 times the radius of its nucleus (1–10 fm), and less than 1/1000 of the wavelength of visible light (400–700 nm).
Q. Why is the atomic radius of k larger than Br?
Since potassium is located at the start of period 3, and bromine at the end of the same period, potassium will have a larger atomic radius than bromine, and thus the largest atomic radius of the four given atoms.
Q. Are there any exceptions to the atomic radius trend?
There is only one exception in the trend of atomic radi along the period. By normal trend atomic radius increases along a period however the atomic radius of noble gases is fgreter than the adjacent halogen atom.
Q. Does atomic radius increase from top to bottom?
The atomic radius of atoms generally increases from top to bottom within a group. As the atomic number increases down a group, there is again an increase in the positive nuclear charge. However, there is also an increase in the number of occupied principle energy levels.
Q. Which is the best reason that the atomic radius generally increases?
the noble gas configuration has been reached. Which is the best reason that the atomic radius generally increases with atomic number in each group of elements? The number of occupied energy levels increases.
Q. Does atomic radius increase down a group?
In general, atomic radius decreases across a period and increases down a group. Down a group, the number of energy levels (n) increases, so there is a greater distance between the nucleus and the outermost orbital. This results in a larger atomic radius.
Q. How do you think the atomic radius will change as electrons are added to a shell?
The gain of an electron adds more electrons to the outermost shell which increases the radius because there are now more electrons further away from the nucleus and there are more electrons to pull towards the nucleus so the pull becomes slightly weaker than of the neutral atom and causes an increase in atomic radius.
Q. How do you think the atomic radii will change as electrons are added to the shell?
As electrons are added to the valence shell, an extra proton (i.e fundamental, positively charged nuclear particle) is added to the element’s nucleus. As electrons add and Z the atomic number increases 1 by 1, nuclear charge WINS, and electronic radii contract.
Q. Why does the atomic radius decrease as electrons are added to a shell quizlet?
Why does the atomic radius decrease from left to right? Electrons enter the same energy level, as protons and electrons are added the force between the protons and electrons increase. The electrons are pulled closer to the nucleus making the atom smaller.
Q. Why does atomic radius increase from top to bottom in a chemical family quizlet?
increases; cause by electron shielding;The number of energy levels increases as you move down a group as the number of electrons increases. Each subsequent energy level is further from the nucleus than the last. Therefore, the atomic radius increases as the group and energy levels increase.
Q. Why does atomic size increase down a group and decrease left to right?
Experiments have shown that the first case is what happens: the increase in nuclear charge overcomes the repulsion between the additional electrons in the valence level. Therefore, the size of atoms decreases as one moves across a period from left to right in the periodic table.
Q. Why does atomic size increase from right to left?
Atomic size gradually decreases from left to right across a period of elements. This is because, within a period or family of elements, all electrons are added to the same shell. However, at the same time, protons are being added to the nucleus, making it more positively charged. Down a group, atomic radius increases.
Q. Which has a larger atomic radius carbon or oxygen?
Carbon belongs to group 14, and oxygen belongs to group 16. As from moving left to right, the atomic radius generally decreases because of the increase of effective nuclear charge. So, carbon has a larger radius than oxygen.
Q. Which has highest electron affinity?
Fluorine D
Q. How do you find electron affinity?
The less valence electrons an atom has, the least likely it will gain electrons. Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far from the nucleus, thus a decrease from its pull.
Q. Which has a larger atomic radius aluminum or oxygen?
This is where the other periodic trends comes into play. As you move down a colomn (a group) of the periodic table, atomic radius increases. This means that Al ‘s atomic radius will be smaller than that of K , but bigger than that of C and O .
Q. Does fluorine or iodine have a larger atomic radius?
Atomic Radius (increases down the group)
| Halogen | Covalent Radius (pm) | Ionic (X-) radius (pm) |
|---|---|---|
| Fluorine | 71 | 133 |
| Chlorine | 99 | 181 |
| Bromine | 114 | 196 |
| Iodine | 133 | 220 |
Q. Is Carbon larger than oxygen?
An oxygen atom has more mass (weight) than a carbon atom because it has more protons and neutrons. Atoms are made up of protons and neutrons, which are heavy, and electrons, which are very light.