Q. How are reactions related to chemical bonds quizlet?
How are reactions related to chemical bonds? Bonds in the reactants are broken, and bonds in the products are formed. Reactants and products form at the same rate.
Q. What happens to chemical bonds during chemical reactions quizlet?
A. What happens to chemical bonds during chemical reactions? During chemical reactions, the chemical bonds between atoms are altered.
Table of Contents
- Q. How are reactions related to chemical bonds quizlet?
- Q. What happens to chemical bonds during chemical reactions quizlet?
- Q. Do chemical reactions break bonds?
- Q. Why do chemicals react with each other and make chemical bonds?
- Q. Which chemical bond is the strongest?
- Q. How many types of chemical bonds are there?
- Q. What are the 5 types of chemical bonds?
- Q. What are the 3 major types of chemical bonds?
- Q. What are the 2 main types of chemical bonds?
- Q. What are two major types of chemical bonds?
- Q. What are the 4 types of bonding?
- Q. How do you identify a chemical bond?
- Q. Which bonds are the strongest and weakest?
- Q. Which types of bonds are the strongest?
- Q. What is the weakest type of bond in chemistry?
- Q. Are covalent or ionic bonds stronger?
- Q. Which bond is the longest?
- Q. Which compound has the shortest C to O bond?
- Q. How do you determine the longest bond?
- Q. What is the shortest covalent bond?
- Q. Which type of covalent bond is the strongest?
- Q. Why is single bond the weakest?
- Q. Why is a triple bond stronger than a single bond?
- Q. Are single covalent bonds strong?
Q. Do chemical reactions break bonds?
Chemical reactions make and break the chemical bonds between molecules, resulting in new materials as the products of the chemical reaction. Breaking chemical bonds absorbs energy, while making new bonds releases energy, with the overall chemical reaction being endothermic or exothermic.
Q. Why do chemicals react with each other and make chemical bonds?
Atoms form chemical bonds with other atoms thereby obtaining the electrons they need to attain a stable electron configuration. The substances used in the beginning of a chemical reaction are called the reactants and the substances found at the end of the reaction are known as the products.
Q. Which chemical bond is the strongest?
Covalent bond
Q. How many types of chemical bonds are there?
There are four types of bonds or interactions: ionic, covalent, hydrogen bonds, and van der Waals interactions.
Q. What are the 5 types of chemical bonds?
The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. A bond between two atoms depends upon the electronegativity difference between the atoms.
Q. What are the 3 major types of chemical bonds?
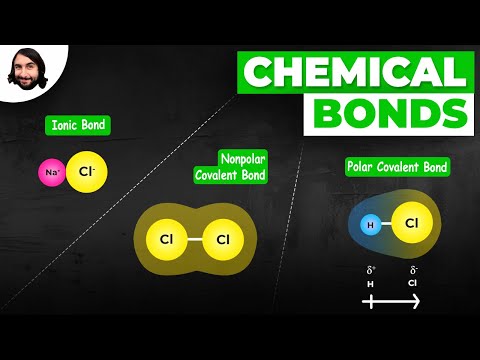
There are three primary types of bonding: ionic, covalent, and metallic.
- Ionic bonding.
- Covalent bonding.
- Metallic bonding.
Q. What are the 2 main types of chemical bonds?
The two main types of bonds formed between atoms are ionic bonds and covalent bonds. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. A covalent bond is formed when atoms share valence electrons.
Q. What are two major types of chemical bonds?
Chemical bonds include covalent, polar covalent, and ionic bonds. Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds. Atoms with large differences in electronegativity transfer electrons to form ions.
Q. What are the 4 types of bonding?
There are four types of chemical bonds essential for life to exist: Ionic Bonds, Covalent Bonds, Hydrogen Bonds, and van der Waals interactions.
Q. How do you identify a chemical bond?
Identifying Types of Bonds
- Look at the chemical formula.
- Identify the elements in the compound.
- Determine if the elements are metals or nonmetals (using a periodic table)
- Metal – Metal = Metallic.
- Metal – Nonmetal = Ionic.
- Nonmetal — Nonmetal = Covalent.
Q. Which bonds are the strongest and weakest?
Of the 4 different types of chemical bonds, covalent bonds are known to be the strongest and the bonds formed via Van der Waals forces are known to be the weakest. The ranking is: Covalent bond > ionic bond > hydrogen bond > Van der Waals forces.
Q. Which types of bonds are the strongest?
Covalent bonds are the strongest (*see note below) and most common form of chemical bond in living organisms. The hydrogen and oxygen atoms that combine to form water molecules are bound together by strong covalent bonds.
Q. What is the weakest type of bond in chemistry?
ionic bond
Q. Are covalent or ionic bonds stronger?
Ionic bonds are generally said to be stronger than covalent bonds. The reason is because ionic bonds are formed by exchange of ions, whereas covalent compounds are formed by sharing of electrons. However, carbon does make strong covalent bonds with hydrogen to form hydrocarbons.
Q. Which bond is the longest?
The longest covalent bond I can find is the bismuth-iodine single bond. The order of bond lengths is single > double > triple. The largest atoms should form the longest covalent bonds. So we look at atoms in the lower right corner of the Periodic Table.
Q. Which compound has the shortest C to O bond?
In CO,C−O bond gets triple bond character in one of the resonating structures. So it has shortest bond length of C−O bond.
Q. How do you determine the longest bond?
The length of the bond is determined by the number of bonded electrons (the bond order). The higher the bond order, the stronger the pull between the two atoms and the shorter the bond length. Generally, the length of the bond between two atoms is approximately the sum of the covalent radii of the two atoms.
Q. What is the shortest covalent bond?
Covalent bonds can either be single, double or triple. A triple bond involves 6 electrons, shared between 2 atoms and is the shortest and strongest.
Q. Which type of covalent bond is the strongest?
sigma bond
Q. Why is single bond the weakest?
The number of component bonds is what determines the strength disparity. It stands to reason that the single bond is the weakest of the three because it consists of only a sigma bond, and the double bond or triple bond consist not only of this type of component bond but also at least one additional bond.
Q. Why is a triple bond stronger than a single bond?
The more electrons that are shared between atoms, the stronger the bond. Single bonds have two electrons shared, double bonds have 4 electrons shared and triple bonds have 6 electrons shared. Thus triple bonds are the strongest.
Q. Are single covalent bonds strong?
A single covalent bond is when only one pair of electrons is shared between atoms. A sigma bond is the strongest type of covalent bond, in which the atomic orbitals directly overlap between the nuclei of two atoms.