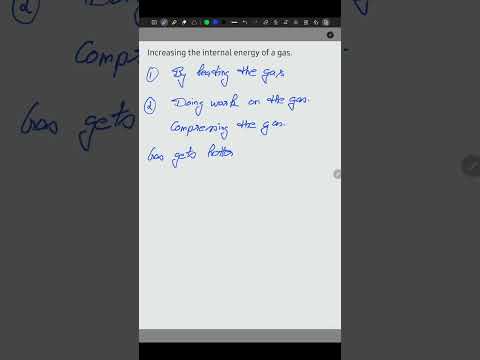
Q. How can the internal energy of a system be increased?
This means that internal energy of a system can be increased by adding heat into the system or by performing work on the system.
Q. Does increasing volume increase internal energy?
The internal energy does not change. If the gas is compressed in such a way so that its pressure remains constant, then by the ideal gas law the temperature drops in proportion to the volume. In this case more energy leaves the system as heat than what you put in as work. The internal energy decreases.
Table of Contents
- Q. How can the internal energy of a system be increased?
- Q. Does increasing volume increase internal energy?
- Q. What is internal energy of air?
- Q. How do you solve for internal energy?
- Q. What causes internal energy?
- Q. What is the symbol for internal energy?
- Q. What is true internal energy?
- Q. What is internal energy example?
- Q. Which has more internal energy?
- Q. Which state has the highest internal energy?
- Q. What is total internal energy?
- Q. Why is internal energy important?
- Q. What is internal energy a function of?
- Q. What are characteristics of internal energy?
- Q. What is internal energy of ideal gas?
- Q. How does internal energy change with temperature?
- Q. Can internal energy negative?
- Q. What are the units of internal energy?
- Q. How do you find the internal energy of a gas?
- Q. How is internal energy related to heat and work?
- Q. What is difference between heat and internal energy?
- Q. Where is internal energy stored?
- Q. What is the relation between heat and energy?
- Q. What is difference between heat and energy?
- Q. What are the 3 laws of energy?
- Q. How do you calculate heat energy?
- Q. What is the formula for energy?
- Q. What are the two main types of energy?
Q. What is internal energy of air?
Each air parcel contains molecules that have internal energy, which when thinking about the atmosphere, is just the kinetic energy of the molecules (associated with molecular rotations and, in some cases, vibrations) and the potential energy of the molecules (associated with the attractive and repulsive forces between …
Q. How do you solve for internal energy?
The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. In equation form, the first law of thermodynamics is ΔU = Q − W. Here ΔU is the change in internal energy U of the system.
Q. What causes internal energy?
The internal energy is the total amount of kinetic energy and potential energy of all the particles in the system. When energy is given to raise the temperature , particles speed up and gain kinetic energy.
Q. What is the symbol for internal energy?
ΔU
Q. What is true internal energy?
Which one is true for internal energy? Explanation: All are correct for internal energy and are part of its property. Explanation: Internal energy does not depend on path.
Q. What is internal energy example?
Internal energy is defined as the energy associated with the random, disordered motion of molecules. For example, a room temperature glass of water sitting on a table has no apparent energy, either potential or kinetic.
Q. Which has more internal energy?
The gas has the highest internal energy because in the liquid and solid phases a lot of energy is bound up in the bonds between atom or molecules.
Q. Which state has the highest internal energy?
Technically however, in the state of plasma molecules would have the highest internal energy, but here, we are only given the choices of solid, liquid, and gas, so gas would be the correct answer.
Q. What is total internal energy?
In chemistry and physics, internal energy (U) is defined as the total energy of a closed system. Internal energy is the sum of potential energy of the system and the system’s kinetic energy.
Q. Why is internal energy important?
This distinction is important because the potential energies between molecules and atoms is important for understanding phase changes, chemical reactions, nuclear reactions, and many more microscopic phenomena. All objects in space exhibit macroscopic and microscopic energy.
Q. What is internal energy a function of?
Internal energy, in thermodynamics, the property or state function that defines the energy of a substance in the absence of effects due to capillarity and external electric, magnetic, and other fields.
Q. What are characteristics of internal energy?
Characteristics of Internal energy: The internal energy of a system is extensive property. It is a state property. The change in internal energy is independent of the path followed. Change in it of a cyclic process is zero.
Q. What is internal energy of ideal gas?
The internal energy of an ideal gas is therefore the sum of the kinetic energies of the particles in the gas. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below.
Q. How does internal energy change with temperature?
When energy is given to raise the temperature , particles speed up and they gain kinetic energy. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy.
Q. Can internal energy negative?
If we have an endothermic reaction heat is gained by the system and the sign of q is positive. Any work done by the system uses energy and the system loses energy, so the sign of w is negative….Internal Energy.
| Energy | Change | Sign |
|---|---|---|
| w | Work is done by the system (expansion) | -w |
Q. What are the units of internal energy?
The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system.
Q. How do you find the internal energy of a gas?
All the heat that was necessary to heat the gas is finally present as internal energy U. Thus, at a temperature T the gas has the following internal energy U: U=cv⋅m⋅T applies in general to perfect gases! Note: Strictly speaking, the specific isochoric heat capacity cv for ideal gases may also be temperature dependent.
Q. How is internal energy related to heat and work?
The relationship between the internal energy of a system and its heat and work exchange with the surroundings is: E = q + w (The form of work will be restricted to gaseous, PV-type for this discussion.)
Q. What is difference between heat and internal energy?
Heat energy This is the energy contained by the body due to its “non-linear” kinetic energy. Internal energy is the total heat content of a system means sum of kinetic and potential energy of a molecule. Heat is the form of temperature which is transferred from one body to any other due to temperature gradient.
Q. Where is internal energy stored?
molecular Level
Q. What is the relation between heat and energy?
Heat is energy transferred between substances or systems due to a temperature difference between them, according to Energy Education. As a form of energy, heat is conserved, i.e., it cannot be created or destroyed. It can, however, be transferred from one place to another.
Q. What is difference between heat and energy?
Energy is the ability of a system to do work and the change of energy, while heat is the energy being transferred.
Q. What are the 3 laws of energy?
Traditionally, thermodynamics has recognized three fundamental laws, simply named by an ordinal identification, the first law, the second law, and the third law. A more fundamental statement was later labelled as the zeroth law, after the first three laws had been established.
Q. How do you calculate heat energy?
The equation for calculating heat energy is q=mCpΔT, where q is the heat variable, m is the mass of the object, Cp is the specific heat constant and ΔT is the temperature change.
Q. What is the formula for energy?
| What is Work, Energy and Power? | |
|---|---|
| Work | |
| Energy | |
| Definition | In physics, we can define energy as the capacity to do work. |
| Formula | For the potential energy the formula is P.E. = mgh |
Q. What are the two main types of energy?
Many forms of energy exist, but they all fall into two basic categories:
- Potential energy.
- Kinetic energy.