De-Broglie’s Explanation of Bohr’s Second Postulate of…
Q. What is the name of James Chadwick atomic model?
This atomic model is known as the quantum mechanical model of the atom. In 1932, James Chadwick bombarded beryllium atoms with alpha particles. An unknown radiation was produced. Chadwick interpreted this radiation as being composed of particles with a neutral electrical charge and the approximate mass of a proton.
Table of Contents
- Q. What is the name of James Chadwick atomic model?
- Q. What are the postulates of the Bohr model and its limitations?
- Q. What is de Broglie equation and how does this equation prove one Bohr’s postulates MW NH 22?
- Q. Which physical quantity is quantized in Bohr’s second postulate?
- Q. Why is angular momentum Quantised?
- Q. Why did Bohr’s quantized angular momentum?
- Q. How did Bohr arrive at the conclusion that electrons are quantized?
- Q. What prevents an atom from being collapse?
- Q. Are all atoms stable in their basic state?
Q. What are the postulates of the Bohr model and its limitations?
The Bohr Model is very limited in terms of size. Poor spectral predictions are obtained when larger atoms are in question. It cannot predict the relative intensities of spectral lines. It does not explain the Zeeman Effect, when the spectral line is split into several components in the presence of a magnetic field.
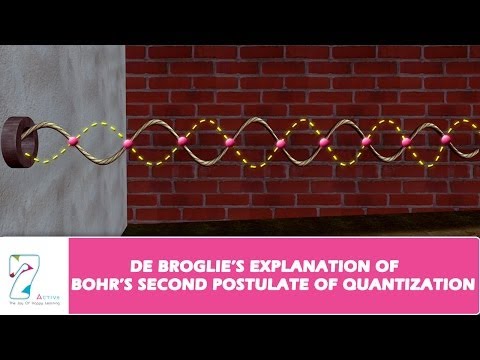
- Electron orbiting in circular orbit can be considered as a particle wave.
- Only those waves propagate and survive which form nodes at terminal point with integer multiple of wavelength(resonant standing waves), thus covering the whole circumferential distance of circular orbit.
Q. What is de Broglie equation and how does this equation prove one Bohr’s postulates MW NH 22?
We know Bohr said that the angular momentum of an electron is an integral multiple of nh/(2π). And in de Broglie’s wave equation, he said the circumference of the path of the electron traveling as a way is n times the wavelength, which is equal to 2πr.
Q. Which physical quantity is quantized in Bohr’s second postulate?
Energy is the physical quantity that is being quantised in postulates os Bohr’s atomic model.
Q. Why is angular momentum Quantised?
What is Angular Momentum of Electron? According to Bohr’s atomic model, the angular momentum of electron orbiting around the nucleus is quantized. He further added that electrons move only in those orbits where angular momentum of an electron is an integral multiple of h/2.
Q. Why did Bohr’s quantized angular momentum?
Bohr was clever enough to find a way to calculate the electron orbital energies in hydrogen. Assuming circular orbits, Bohr proposed that the angular momentum L of an electron in its orbit is quantized, that is, it has only specific, discrete values.
Q. How did Bohr arrive at the conclusion that electrons are quantized?
With his model, Bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.
Q. What prevents an atom from being collapse?
The balance of kinetic and potential energy in an atom is what keeps its electrons from collapsing into the nucleus.
Q. Are all atoms stable in their basic state?
Answer: option galat likha hai. All the atoms are stable in their basic state.