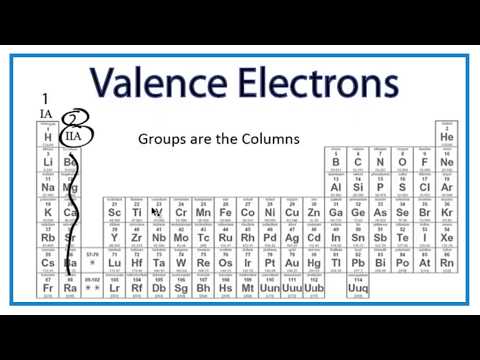
Q. How do I find the valence of an atom?
For neutral atoms, the number of valence electrons is equal to the atom’s main group number. The main group number for an element can be found from its column on the periodic table. For example, carbon is in group 4 and has 4 valence electrons. Oxygen is in group 6 and has 6 valence electrons.
Q. In which part of the atom are valence electrons found?
outermost shell
Table of Contents
- Q. How do I find the valence of an atom?
- Q. In which part of the atom are valence electrons found?
- Q. What is Atom Valence?
- Q. Is carbon 14 a metal or nonmetal?
- Q. What is the Valency of silver?
- Q. What is Valency simple words?
- Q. How can we find Valency with example?
- Q. What is difference between oxidation number and Valency?
- Q. What do you mean by oxidation number?
- Q. Is oxidation a charge number?
Q. What is Atom Valence?
Valence, also spelled valency, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Introduced in 1868, the term is used to express both the power of combination of an element in general and the numerical value of the power of combination.
Q. Is carbon 14 a metal or nonmetal?
Introduction
| Element | Symbol | Classification |
|---|---|---|
| Carbon | C | Non-metal |
| Silicon | Si | Metalloid |
| Germanium | Ge | Metalloid |
| Tin | Sn | Metal |
Q. What is the Valency of silver?
+ 1
Q. What is Valency simple words?
Valency is the combining power of an element. Elements in the same group of the periodic table have the same valency. The valency of an element is related to how many electrons are in the outer shell.
Q. How can we find Valency with example?
For example, oxygen has six valence electrons, but its valency is 2. Some elements may have more than one combining power (or valency), while others have just one. For example, H →1; Mg→2; Al→3; C→4; N→3, and 5; P→3 and 5; O→2; S→ 2, 4 and 6; Cl→ 1; and Ne→0.
Q. What is difference between oxidation number and Valency?
The valency and oxidation state may have the same values or different values. Valency is the number of electrons present in the outermost shell of a particular element whereas oxidation state is the number of electrons that an element in a particular compound has lost or gained.
Q. What do you mean by oxidation number?
Oxidation number, also called oxidation state, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom.
Q. Is oxidation a charge number?
An atom’s oxidation number (or oxidation state) is the imaginary charge that the atom would have if all of the bonds to the atom were completely ionic. Oxidation numbers can be assigned to the atoms in a reaction using the following guidelines: An atom of a free element has an oxidation number of 0.