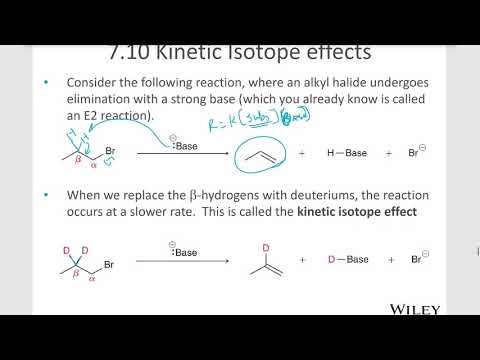
Q. How do isotopes affect chemical reactions?
In summary, the greater the mass the more energy is needed to break bonds. A heavier isotope forms a stronger bond. The resulting molecule has less of a tendency to dissociate. The increase in energy needed to break the bond results in a slower reaction rate and the observed isotope effect.
Q. What are uses of isotopes?
Medical Applications
Table of Contents
- Q. How do isotopes affect chemical reactions?
- Q. What are uses of isotopes?
- Q. What do isotopes tell us?
- Q. What is the relationship between an element and an isotope?
- Q. What was wrong with Dalton?
- Q. What did Dalton’s theory of matter propose?
- Q. Did John Dalton believe in God?
- Q. Did John Dalton win any awards?
- Q. What evidence did Dalton use to argue for the existence of atoms?
- Q. What is the John Dalton Award?
| Isotope | Use |
|---|---|
| 99mTc* | brain, thyroid, liver, bone marrow, lung, heart, and intestinal scanning; blood volume determination |
| 131I | diagnosis and treatment of thyroid function |
| 133Xe | lung imaging |
| 198Au | liver disease diagnosis |
Q. What do isotopes tell us?
It is the electrons that determine the chemical behaviour of a particular element. Isotopes of an element share the same number of protons but have different numbers of neutrons. This means that all three isotopes have different atomic masses (carbon-14 being the heaviest), but share the same atomic number (Z=6).
Q. What is the relationship between an element and an isotope?
Isotopes are atoms of the same element that have different numbers of neutrons but the same number of protons and electrons. The difference in the number of neutrons between the various isotopes of an element means that the various isotopes have different masses.
Q. What was wrong with Dalton?
Drawbacks of Dalton’s Atomic Theory The indivisibility of an atom was proved wrong: an atom can be further subdivided into protons, neutrons and electrons. However an atom is the smallest particle that takes part in chemical reactions. According to Dalton, the atoms of same element are similar in all respects.
Q. What did Dalton’s theory of matter propose?
Dalton’s atomic theory proposed that all matter was composed of atoms, indivisible and indestructible building blocks. While all atoms of an element were identical, different elements had atoms of differing size and mass.
Q. Did John Dalton believe in God?
He remained a faithful Quaker all of his life, living modestly. In 1810, he declined an invitation to become a member of the Royal Society. In 1822, he was elected without his knowledge. In 1826, he was awarded the Society’s Royal Medal for his Atomic Theory.
Q. Did John Dalton win any awards?
Royal Medal
Q. What evidence did Dalton use to argue for the existence of atoms?
Dalton did many experiments that provided evidence for the existence of atoms. For example: He investigated pressure and other properties of gases, from which he inferred that gases must consist of tiny, individual particles that are in constant, random motion.