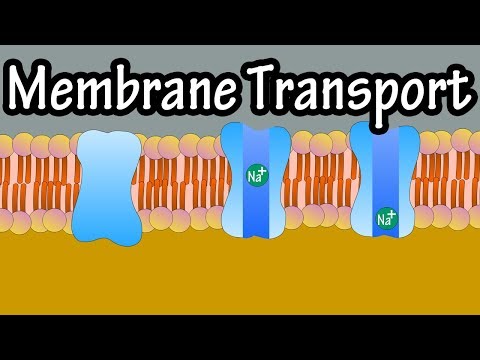
Q. How do molecules move through a membrane?
Explanation: Molecules move across the plasma/cell membrane through diffusion. If they are small enough, usually, the easiest way for them to move is through diffusion. This means that they will move from a region of higher concentration to a region of lower concentration.
Q. What molecules can and Cannot pass through the membrane?
Cell membranes serve as barriers and gatekeepers. They are semi-permeable, which means that some molecules can diffuse across the lipid bilayer but others cannot. Small hydrophobic molecules and gases like oxygen and carbon dioxide cross membranes rapidly.
Table of Contents
- Q. How do molecules move through a membrane?
- Q. What molecules can and Cannot pass through the membrane?
- Q. What type of molecule has the most difficult time passing through the membrane?
- Q. Which molecule is smaller starch or iodine?
- Q. What type of indicator is iodine?
- Q. Why Iodine is used for starch test?
- Q. What happens when iodine comes in contact with starch?
- Q. Why is starch used as an indicator?
- Q. What is starch indicator called?
- Q. What is the best indicator for starch?
- Q. Is starch an indicator?
- Q. What is Starch Indicator formula?
- Q. Why is starch solution added only at the end of titration?
- Q. How do you make starch indicator?
- Q. How do you make a 1% starch indicator?
- Q. How do you make a 2% starch solution?
- Q. What is the original starting color for the starch indicator?
- Q. Why iodometric titrations are done in dark?
- Q. What is the Colour change during titration of BOD experiment with na2s2o3 before adding starch?
- Q. What is the end point of this titration?
Q. What type of molecule has the most difficult time passing through the membrane?
Polar and charged molecules have much more trouble crossing the membrane. Polar molecules can easily interact with the outer face of the membrane, where the negatively charged head groups are found, but they have difficulty passing through its hydrophobic core.
Q. Which molecule is smaller starch or iodine?
From the results of this experiment, it is obvious that glucose and iodine (potassium iodide) has smaller molecular size than starch. Because starch had larger molecular size, the dialysis tubing was not permeable to it (it didn’t allow it to readily pass through the pores of its membrane).
Q. What type of indicator is iodine?
Starch as an indicator Starch forms a very dark blue-black complex with triiodide. However, the complex is not formed if only iodine or only iodide (I−) is present. The colour of the starch complex is so deep, that it can be detected visually when the concentration of the iodine is as low as 20 µM at 20 °C.
Q. Why Iodine is used for starch test?
Amylose in starch is responsible for the formation of a deep blue color in the presence of iodine. The iodine molecule slips inside of the amylose coil. This makes a linear triiodide ion complex with is soluble that slips into the coil of the starch causing an intense blue-black color.
Q. What happens when iodine comes in contact with starch?
The starch undergoes a chemical reaction with the iodine. This reactions turns starch a dark blue black color, This is an effective indicator for the presence of starch.
Q. Why is starch used as an indicator?
Starch is a viable indicator in the titration process because it turns deep dark blue when iodine is present in a solution. When starch is heated in water, decomposition occurs and beta-amylose is produced. Vitamin C converts iodine to iodide, so the starch mixture won’t turn blue until all the vitamin C is used up.
Q. What is starch indicator called?
Iodine Test
Q. What is the best indicator for starch?
Starch reacts with Iodine in the presence of Iodide ion to form an intensely colored blue complex, which is visible at very low concentrations of Iodine, making it a very good indicator in both direct and indirect lodometric titrations.
Q. Is starch an indicator?
Starch is a indicator in the iodometric titration and it turns deep dark blue when iodine is present in a solution. The starch under warming condition forms amylose and amylo pectins which combine with iodine to produce dark blue color.In absence of iodide ion starch indicator is colorless.
Q. What is Starch Indicator formula?
The basic chemical formula of the starch molecule is (C6H10O5)n. Starch is a polysaccharide comprising glucose monomers joined in α 1,4 linkages. The simplest form of starch is the linear polymer amylose; amylopectin is the branched form.
Q. Why is starch solution added only at the end of titration?
Starch is only added towards the end when the iodine solution is lower. Since starch is a complex molecule, it traps the iodine molecules when exposed to high concentrations of it. The trapped iodine molecules do not react with Na S O and results in a diffuse endpoint.
Q. How do you make starch indicator?
To prepare starch indicator solution, add 1 gram of starch (either corn or potato) into 10 mL of distilled water, shake well, and pour into 100 mL of boiling, distilled water. Stir thoroughly and boil for a 1 minute. Leave to cool down. If the precipitate forms, decant the supernatant and use as the indicator solution.
Q. How do you make a 1% starch indicator?
** To prepare the 1% starch solution, mix 1 / 2 teaspoon of soluble starch (cornstarch or potato starch) with a small amount of water. Stir this mixture into 100mL of boiling distilled water. Boil for 1 minute and then cool. Put in marked dropper bottle.
Q. How do you make a 2% starch solution?
Protocol
- Put 500ml of distilled water to the beaker on the magnetic stirrer.
- Weigh 10.20g of starch.
- Empty gradually the container with starch into the spinning water.
- Prepare the bottle with lables “Starch. 2% Stock Solution” and “Shake before use”
- Put the solution into the bottle.
- Put the bottle to the fridge for storage.
Q. What is the original starting color for the starch indicator?
The indicator that is usually chosen for titrations involving iodine (triiodide) is starch. Starch forms a dark blue complex with iodine.
Q. Why iodometric titrations are done in dark?
The reaction mixture should be kept in the dark before titration because light accelerates a side reaction in which iodide ions are oxidized to iodine by atmospheric oxygen.
Q. What is the Colour change during titration of BOD experiment with na2s2o3 before adding starch?
The starch indicator is added to the solution near the end of the titration, at the point where dilute iodine imparts a pale yellow color to the solution. There are two reasons why the indicator is not added at the beginning of the titration when the iodine concentration is high.
Q. What is the end point of this titration?
End Point. end point: the point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been added to a solution.