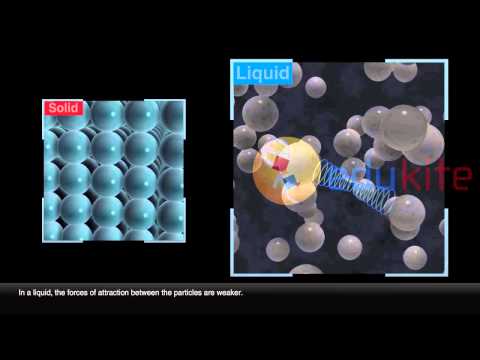
In liquids, particles are quite close together and move with random motion throughout the container. The particles are held together too strongly to allow movement from place to place but the particles do vibrate about their position in the structure.
Q. How do molecules in solid liquid and gas move?
gas vibrate and move freely at high speeds. liquid vibrate, move about, and slide past each other. solid vibrate (jiggle) but generally do not move from place to place.
Table of Contents
- Q. How do molecules in solid liquid and gas move?
- Q. Do molecules move faster in a solid liquid or gas?
- Q. How do molecules move in a solid?
- Q. How do you prove that matter is made up of particles based on the activity?
- Q. What happens to molecules when a solid changes to a liquid?
- Q. Why do molecules move?
- Q. How do molecules move in a liquid?
- Q. Why do solids and liquids behave differently Brainly?
- Q. Which of the following describes the molecules in a solid?
- Q. What is the relationship of the arrangement of molecules in solid liquid and gas with the speed of sound?
- Q. How does the movement of water molecules change when water goes from liquid to gas?
- Q. How are molecules move in a solid liquid and gas?
- Q. How are particles able to move in a liquid?
- Q. Why do particles move faster in a gas than a solid?
- Q. How are molecules attracted to each other in a liquid?
Q. Do molecules move faster in a solid liquid or gas?
Particles in a gas state move much faster than a liquid.
Q. How do molecules move in a solid?
In a solid, the atoms are very attracted to one another. The atoms vibrate but stay in fixed positions because of their strong attractions for one another. An increase in the motion of the atoms competes with the attraction between atoms and causes them to move a little further apart.
Q. How do you prove that matter is made up of particles based on the activity?
Answer Expert Verified
- take crystals of KMNO4 and dissolved them in 100 ml of water.
- take 10 ml from this solution and dissolve in 90 ml of water.
- take 10 ml from this solution and dissolve in 90 ml of water.
- repeat this step 4-5 times.
- you will observe that colored water still after 4-5 times.
Q. What happens to molecules when a solid changes to a liquid?
Solid to a Liquid This change of state is called melting. By adding energy to the molecules in a solid the molecules begin to move quicker and can break away from the other molecules. This happens slowly as each and every single molecule in the substance has to get enough energy to move quicker.
Q. Why do molecules move?
Molecules are in constant motion. Molecules are so much smaller than us that what gives them energy is actually heat. At room temperature there is enough heat energy around us that molecules constantly move. When molecules are in the gas phase, like in the air we breathe, they move around a lot.
Q. How do molecules move in a liquid?
Q. Why do solids and liquids behave differently Brainly?
Solids, liquids and gases are different mainly because of their lattice arrangements and the cohesive forces between the molecules. 2) Liquids — Liquid molecules are farther apart compared to solid molecules. The cohesive forces between liquid molecules are also weaker compared to solids therefore liquids flow.
Q. Which of the following describes the molecules in a solid?
Answer: D: The molecules are compact and close together.
Q. What is the relationship of the arrangement of molecules in solid liquid and gas with the speed of sound?
Sound travels more quickly through solids than through liquids and gases because the molecules of a solid are closer together and, therefore, can transmit the vibrations (energy) faster. Sound travels most slowly through gases because the molecules of a gas are farthest apart.
Q. How does the movement of water molecules change when water goes from liquid to gas?
As that liquid water is further heated, it evaporates and becomes a gas—water vapor. These changes between states (melting, freezing, and evaporating) happen because as the temperature either increases or decreases, the molecules in a substance begin to speed up or slow down.
Q. How are molecules move in a solid liquid and gas?
The particles in a solid are tightly packed and locked in place. The particles in a liquid are close together (touching) but they are able to move/slide/flow past each other. The particles in a gas are fast moving and are able to spread apart from each other.
Q. How are particles able to move in a liquid?
Individual particles in liquids are able to move all through the mass of liquid. The motion of particles in a liquid is kinetic energy. When a liquid gets warm, the particles move faster. The particles have more kinetic energy.
Q. Why do particles move faster in a gas than a solid?
The particles are moving much faster than in a solid. Gases. When you add even more energy to the substance, you increase the kinetic energy of those particles so much, that they lose their state form, becoming a gas.
Q. How are molecules attracted to each other in a liquid?
The molecules of a liquid are attracted to each other, but move more freely and past one another. Accordingly, what is the motion of particles in a liquid? Individual particles in liquids are able to move all through the mass of liquid. The motion of particles in a liquid is kinetic energy.