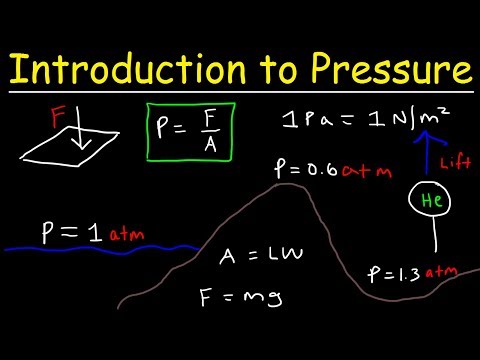
Q. How do you calculate force with pressure?
Pressure and force are related, and so you can calculate one if you know the other by using the physics equation, P = F/A. Because pressure is force divided by area, its meter-kilogram-second (MKS) units are newtons per square meter, or N/m2.
Q. How do you find the pressure of a cuboid?
To do this, just click on the iron cuboid that is in the tray. Now calculate the Area and Pressure and note the values in the respective boxes. (Area = Length x Breadth) (Pressure = Thrust / Area, where Thrust = Mass x Acceleration due to gravity)
Table of Contents
- Q. How do you calculate force with pressure?
- Q. How do you find the pressure of a cuboid?
- Q. How do you find pressure from area?
- Q. How do you find the pressure?
- Q. Are pressure and volume directly related?
- Q. What happens to volume when pressure increases?
- Q. Is there a relationship between temperature and pressure?
- Q. What is the relation between temperature and pressure class 7?
- Q. What is the relationship between temperature and pressure in Earth’s atmosphere?
- Q. Why does pressure decrease with temperature?
- Q. How does temperature affect pressure?
- Q. What happens to the air pressure when you go up in the atmosphere?
Q. How do you find pressure from area?
Calculating force and area using the pressure equation
- First convert 150 kPa to Pa:
- 150 × 1,000 = 150,000.
- Next substitute the values into the equation:
- force normal to a surface area = pressure × area of that surface.
- force = 150,000 × 180.
- force = 27,000,000 N.
Q. How do you find the pressure?
Section Summary
- Pressure is the force per unit perpendicular area over which the force is applied. In equation form, pressure is defined as. P=FA P = F A .
- The SI unit of pressure is pascal and 1 Pa=1 N/m2 1 Pa = 1 N/m 2 .
Q. Are pressure and volume directly related?
Key Concepts and Summary The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles’s law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle’s law).
Q. What happens to volume when pressure increases?
Boyle found that when the pressure of gas at a constant temperature is increased, the volume of the gas decreases. this relationship between pressure and volume is called Boyle’s law. So, at constant temperature, the answer to your answer is: the volume decreases in the same ratio as the ratio of pressure increases.
Q. Is there a relationship between temperature and pressure?
We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then P and T are directly proportional (again, when volume and moles of gas are held constant); if the temperature on the kelvin scale increases by a certain factor, the gas pressure increases by the same factor.
Q. What is the relation between temperature and pressure class 7?
Answer: Distribution of air pressure is influenced by the temperature of the area: Where temperature is high the air gets heated and rises. This creates a low pressure area.
Q. What is the relationship between temperature and pressure in Earth’s atmosphere?
The density and, therefore, pressure of atmospheric gases are related to their temperature. As temperature increases, gas molecules move faster and occupy a greater volume. This reduces their density which, in turn, reduces their pressure. Warm gases in the atmosphere are less dense than cold gases in the atmosphere.
Q. Why does pressure decrease with temperature?
In order to achieve the same pressure, the molecules in the more dense gas must move slower. This leads directly to a decrease in temperature. If, instead, the density of the gas increases and the pressure is to remain constant, there must be a decrease in temperature.
Q. How does temperature affect pressure?
The temperature of the gas is proportional to the average kinetic energy of its molecules. Faster moving particles will collide with the container walls more frequently and with greater force. This causes the force on the walls of the container to increase and so the pressure increases.
Q. What happens to the air pressure when you go up in the atmosphere?
As you travel away from the Earth’s surface, the atmosphere expands the further you go. As the atmosphere expands the further you get from the Earth’s surface, it becomes less dense and air pressure decreases. As you increase altitude (distance from Earth’s surface) in an airplane, air pressure changes.