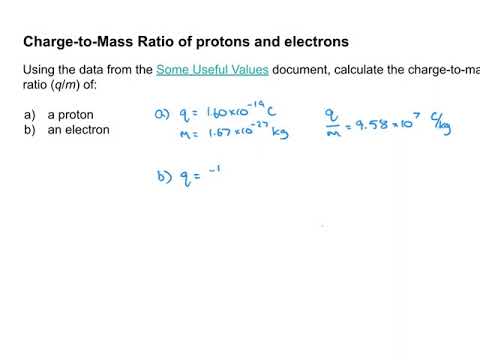
Q. How do you calculate the charge mass ratio of a proton?
Using a proton charge 1.602*10^-19 coulombs is equal to proton mass 1.673*10^-27 Kg. Therefore One coulomb is equal to 1.044*10^-8Kg.
Q. What is the ratio of charge of an electron to the mass of proton?
The ratio of charge/mass of a proton is 9.58 x 104C/g and that of the electron is 1.76 x 108C/g.
Table of Contents
- Q. How do you calculate the charge mass ratio of a proton?
- Q. What is the ratio of charge of an electron to the mass of proton?
- Q. How is charge mass ratio calculated?
- Q. Which subatomic particle has the ratio of charge and mass?
- Q. What is the charge to mass ratio of electrons?
- Q. Which particle has constant charge to mass ratio for all matter?
- Q. Why is the charge mass ratio of anode rays not constant?
- Q. Who determine the charge to mass ratio of electron in 1897?
- Q. Why is the charge to mass ratio of an electron important?
- Q. Is the charge to mass ratio of an electron constant?
- Q. What is the charge to mass ratio of cathode rays?
- Q. Does cathode rays have mass and charge?
Q. How is charge mass ratio calculated?
Charge to Mass Ratio of Electron
- m = mass of an electron in kg = 9.10938356 × 10-31 kilograms.
- e = magnitude of the charge of an electron in coulombs = 1.602 x 10-19 coulombs.
Q. Which subatomic particle has the ratio of charge and mass?
electron
Q. What is the charge to mass ratio of electrons?
Charge by Mass Ratio of an Electron = 1.602 10-19 coulombs.
Q. Which particle has constant charge to mass ratio for all matter?
In conclusion 1.045*10-8 Kg of matter contains a maximum of 1 coulomb of positive charge. This means 1Kg of matter contains a maximum of 95698925 coulombs of positive charge. This constant is approximately equal to a proton mass to charge ratio. It is also approximately equal to a plank mass.
Q. Why is the charge mass ratio of anode rays not constant?
Why does the charge to mass ratio of anode rays depend on the gas from which it originates? The different gases have different types of positive rays,that contain particles of different mass and charge. Therefore, the charge to mass ratio of anode depends on the gas. This ratio is not constant.
Q. Who determine the charge to mass ratio of electron in 1897?
J.J. Thomson
Q. Why is the charge to mass ratio of an electron important?
Answer: Knowing the charge to mass ratio allows us to calculate the mass by measuring charge effects. Since the force on a charged particle is proportional to its charge, the deflection of a beam of charged particles will be the same for all particles with the same charge to mass ratio.
Q. Is the charge to mass ratio of an electron constant?
An electron is a fundamental particle. All electrons have the same mass (9.1 x 10^-31 Kg) and charge (- 1.6 x 10^-19 Coul) so that e/m is constant. (Values of mass and charge are rounded off for this answer).
Q. What is the charge to mass ratio of cathode rays?
By definition, one coulomb is the charge carried by a current of one ampere that flows for one second: 1 C = 1 amp-s. When Thomson’s data are converted to SI units, the charge-to-mass ratio of the particles in the cathode-ray beam is about 108 coulomb per gram.
Q. Does cathode rays have mass and charge?
When electric fields are applied to the cathode rays, they are deflected upward. Thus he established the electric charge. Thus the cathode rays have both mass and charge.