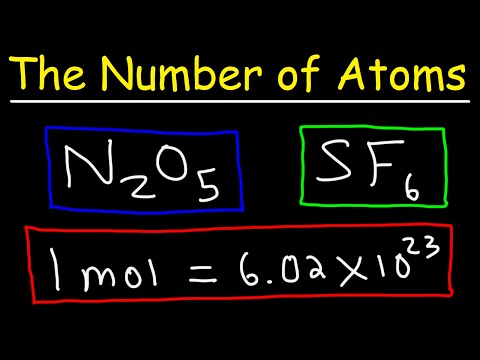
To calculate the number of atoms in a sample, divide its weight in grams by the amu atomic mass from the periodic table, then multiply the result by Avogadro’s number: 6.02 x 10^23.
Q. Which compound do atoms form bonds by sharing electrons?
covalent bond
Table of Contents
- Q. Which compound do atoms form bonds by sharing electrons?
- Q. How do you know how many atoms are in a compound?
- Q. How do you find the mass of one element in a compound?
- Q. What is smaller than a gluon?
- Q. How many atoms are in a human cell?
- Q. What is the smallest chip in the world?
- Q. What is the smallest electronic device?
- Q. How many atoms are in a grain of sand?
- Q. Is Salt smaller than sand?
- Q. Is there more atoms in a grain of sand?
- Q. How many atoms are in a pea?
- Q. How many atoms are in a full stop?
- Q. How many atoms can fit on a pinhead?
- Q. What is the smallest number of atoms we can see?
- Q. How many atoms are in a period?
Q. How do you know how many atoms are in a compound?
Explanation:
- Mass → Moles and Moles → Atoms.
- 196.967 u .
- So, if you are given the mass of an element, you use the periodic table to find its molar mass, and multiply the given mass by the reciprocal of the molar mass.
- Once you have moles, multiply by Avogadro’s number to calculate the number of atoms.
Q. How do you find the mass of one element in a compound?
The characteristic molar mass of an element is simply the atomic mass in g/mol. However, molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant (1 g/mol). To calculate the molar mass of a compound with multiple atoms, sum all the atomic mass of the constituent atoms.
Q. What is smaller than a gluon?
Leptons: electron, electron neutrino, muon, muon neutrino, tau, tau neutrino. The elementary bosons (force carrying particles with integer spin ) are: Gluon, W and Z, photon. In what you said, the electron is a fundamental particle but the neutron and proton are not.
Q. How many atoms are in a human cell?
100 trillion atoms
Q. What is the smallest chip in the world?
Today, IBM is unveiling the world’s first 2 nanometer (nm) transistor-based chip, the smallest yet, which will unlock greater performance and energy efficiency over the next decade.
Q. What is the smallest electronic device?
Scientists have developed the world’s smallest computer a device that measures just 0.3 millimetres and could help find new ways to monitor and treat cancer Previous systems, including the 2x2x4 millimetre Michigan Micro Mote, retain their programming and data even when they are not externally powered.
Q. How many atoms are in a grain of sand?
In each molecule of quartz we have 3 atoms (1 silicon and 2 oxygen), so this means there are roughly 2*10 19 atoms in a small grain of sand.
Q. Is Salt smaller than sand?
Physical Separation of Salt and Sand In other words, sand is slightly heavier than salt.
Q. Is there more atoms in a grain of sand?
For a typical grain of sand (size 1mm) made of Si02 , the estimate is 1019 atoms… If a grain is a fraction of mg, 1 g of grain has truly more atoms than stars in the universe.
Q. How many atoms are in a pea?
A mole of any element contains 602, 000, 000, 000, 000, 000, 000, 000 atoms of that element, that is 602 sexillion US, 602 trilliard UK or 6.02 x 1023 atoms….
| Exponent | Value | Analogy |
|---|---|---|
| 106 | 1 000 000 | 10 fridgefuls of peas – an average bedroom |
| 107 | 10 000 000 | Average house full from basement to attic with peas |
Q. How many atoms are in a full stop?
Atoms are so tiny that even the full stop at the end of this sentence has a width of around 20 million atoms. Inside each atom are even smaller particles, called subatomic particles. These include a nucleus, which contains protons and neutrons, and electrons that whizz around the nucleus.
Q. How many atoms can fit on a pinhead?
five million million
Q. What is the smallest number of atoms we can see?
Originally Answered: What is the smallest amount of atoms it would take to form something barely visible to the naked eye? The smallest objects that the naked or unaided human eye can see are about 0.1 mm long. That’s one-tenth of one-mm, the distance you can put your thumb and forefinger close while not touching.
Q. How many atoms are in a period?
nc=6.023×1023atoms/mole⋅2.01g/cm312.01g/mole(π(0.052cm)2(0.01cm)4)=2.14×1018atoms. Thus, there are approximately 2.14×1018 atoms in the period; that is, there are a billion, billion carbon atoms in the period.