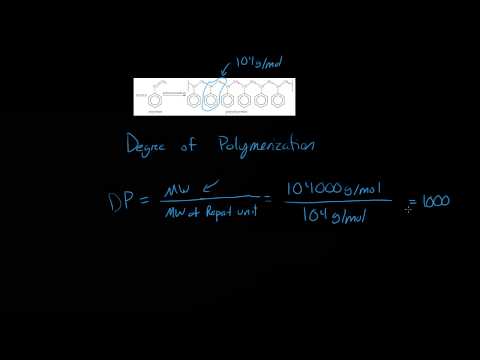
Divide the molecular weight of the polymer by the molecular weight of the monomer unit to calculate the degree of polymerization. If the molecular mass of tetrafluoroethylene is 120,000, its degree of polymerization is 120,000 / 100 = 1,200.
Table of Contents
- Q. How do you calculate the average molecular weight of a polymer?
- Q. What is number average molecular weight of a polymer?
- Q. Why weight average molecular weight is greater than number average molecular weight?
- Q. Why is it difficult to determine a molecular weight for a polymer?
- Q. What is Z average molecular weight?
- Q. How do I calculate molecular weight?
- Q. What is the Z average?
- Q. What is the average molecular weight of the mixture?
- Q. How do you calculate apparent molecular weight?
- Q. How do you find the molecular weight of a log?
- Q. How do you calculate the average molecular weight of a gas mixture?
- Q. What is the molecular weight of the gas?
- Q. What is molecular weight of n2?
- Q. How do you calculate the weight of a gas?
- Q. What is the molecular weight of an unknown gas?
- Q. What is the weight of domestic cylinder?
- Q. How many pounds does 5 gallons of gas weigh?
- Q. What is the weight of 1 gallon of gasoline?
- Q. How much does a gallon of 87 octane weight?
- Q. How much does 1 gallon of unleaded gas weigh?
- Q. What weighs more water or gasoline?
- Q. How heavy is a gallon of milk?
- Q. How much gas do you waste per mile?
- Q. How do you calculate miles per gallon of gas?
- Q. How far will 12 gallons of gas get you?
- Q. How much will 10 dollars of gas get you?
- Q. What is a good amount for gas money?
- Q. How many gallons of gas is ?
- Q. How many miles is a 2 hour drive?
Q. How do you calculate the average molecular weight of a polymer?
The distribution of molecular weights in a polymer sample is often described by the ratio of the weight average molecular weight to the number average molecular weight. In this case the ratio is 000 = 1.063. This ratio is the Polydispersity Index (or PDI)….
Table of Contents
- Q. How do you calculate the average molecular weight of a polymer?
- Q. What is number average molecular weight of a polymer?
- Q. Why weight average molecular weight is greater than number average molecular weight?
- Q. Why is it difficult to determine a molecular weight for a polymer?
- Q. What is Z average molecular weight?
- Q. How do I calculate molecular weight?
- Q. What is the Z average?
- Q. What is the average molecular weight of the mixture?
- Q. How do you calculate apparent molecular weight?
- Q. How do you find the molecular weight of a log?
- Q. How do you calculate the average molecular weight of a gas mixture?
- Q. What is the molecular weight of the gas?
- Q. What is molecular weight of n2?
- Q. How do you calculate the weight of a gas?
- Q. What is the molecular weight of an unknown gas?
- Q. What is the weight of domestic cylinder?
- Q. How many pounds does 5 gallons of gas weigh?
- Q. What is the weight of 1 gallon of gasoline?
- Q. How much does a gallon of 87 octane weight?
- Q. How much does 1 gallon of unleaded gas weigh?
- Q. What weighs more water or gasoline?
- Q. How heavy is a gallon of milk?
- Q. How much gas do you waste per mile?
- Q. How do you calculate miles per gallon of gas?
- Q. How far will 12 gallons of gas get you?
- Q. How much will 10 dollars of gas get you?
- Q. What is a good amount for gas money?
- Q. How many gallons of gas is ?
- Q. How many miles is a 2 hour drive?
| Number of Molecules | Mass of each Molecule |
|---|---|
| 1 | 200,000 |
Q. What is number average molecular weight of a polymer?
The number average molecular weight is defined as the total weight of polymer divided by the total number of molecules.
Q. Why weight average molecular weight is greater than number average molecular weight?
Because of this, polymer molecular weights are usually given as averages. Since larger molecules in a sample weigh more than smaller molecules, the weight average Mw is necessarily skewed to higher values, and is always greater than Mn.
Q. Why is it difficult to determine a molecular weight for a polymer?
3.1. 1.1 Polypropylene Molecular Weight Characterization. Polymer molecular weight is defined as a distribution rather than a specific number because polymerization occurs in such a way to produce different chain lengths.
Q. What is Z average molecular weight?
The z-average molar mass is the third moment or third power average molar mass, which is calculated by. The z-average molar mass can be determined with ultracentrifugation. The melt elasticity of a polymer is dependent on Mz.
Q. How do I calculate molecular weight?
Sample Molecular Weight Calculation Using the periodic table of the elements to find atomic weights, we find that hydrogen has an atomic weight of 1, and oxygen’s is 16. In order to calculate the molecular weight of one water molecule, we add the contributions from each atom; that is, 2(1) + 1(16) = 18 grams/mole.
Q. What is the Z average?
The Z average is the intensity weighted mean hydrodynamic size of the ensemble collection of particles measured by dynamic light scattering (DLS).
Q. What is the average molecular weight of the mixture?
The Average Molecular Weight of a mixture is computed from the molar composition and the molecular weight. It is a weighted average — the molecular weights are averaged using the mole fractions as weights. EXAMPLE: Calculate the average molecular weight of air. Assume air is 79 mole % nitrogen, 21 mole % oxygen.
Q. How do you calculate apparent molecular weight?
The apparent molecular weight of a gas mixture is equal to the sum of the mole fraction times the molecular weight of each component.
Q. How do you find the molecular weight of a log?
Use a graphing program, plot the log (MW) as a function of Rf. Generate the equation y = mx + b, and solve for y to determine the MW of the unknown protein. Run the standards and samples on an SDS-PAGE gel. Process the gel with the desired stain and then destain to visualize the protein bands.
Q. How do you calculate the average molecular weight of a gas mixture?
The term “average molecular weight” is often used to describe the molar mass of a gas mixture. Find the average molar mass of dry air whose volume-composition is O2 (21%), N2 (78%) and Ar (1%). The average molecular weight is the mole-fraction-weighted sum of the molecular weights of its components.
Q. What is the molecular weight of the gas?
The molecular weight ( molar mass ) of any gas is the mass of one particle of that gas multiplied by Avogadro’s number (6.02 x 1023). Knowing the molar mass of an element or compound can help us stoichiometrically balance a reaction equation.
Q. What is molecular weight of n2?
28.0134 g/mol
Q. How do you calculate the weight of a gas?
1 Answer
- PV=nRT. We can modify this law in terms of the molar mass ( MM ).
- n=mMM. If we plug the expression of number of mole in the expression of the ideal gas law we get:
- PV=mMMRT. therefore, the molecular mass expression can be written as:
- MM=mRTPV.
Q. What is the molecular weight of an unknown gas?
First the ideal gas law will be used to solve for the moles of unknown gas (n). Then the mass of the gas divided by the moles will give the molar mass. Step 2: Solve. Now divide g by mol to get the molar mass.
Q. What is the weight of domestic cylinder?
The gross weight of the cylinder should be arrived at by adding tare weight and the amount of 14.2 kg LPG. For example, if the tare weight printed on the cylinder is 15.2 kg, a full cylinder with 14.2 kg LPG should have a gross weight of 29.4 kg.
Q. How many pounds does 5 gallons of gas weigh?
So, the answer here is, of course, relative, as weight is contingent on other factors. But, to give you an average figure, a U.S. gallon of gasoline weighs about 6 pounds according to the Science and Technology Desk Reference. For comparison, a U.S. gallon of water weighs about 8.4 pounds.
Q. What is the weight of 1 gallon of gasoline?
about 6.3 pounds
Q. How much does a gallon of 87 octane weight?
6.216 pounds
Q. How much does 1 gallon of unleaded gas weigh?
six pounds
Q. What weighs more water or gasoline?
Gasoline floats on water which means that water has a higher density than gasoline. The weight of one gallon of commonly used fuel, like that of gasoline, is six pounds. To put this into context with water, a gallon of water weighs about 8.4 pounds.
Q. How heavy is a gallon of milk?
8.6 lb
Q. How much gas do you waste per mile?
Gas price per mile for common vehicles
| Vehicle | Miles per gallon | Gas price, per mile |
|---|---|---|
| PT Cruiser | 22 | $0.13 |
| Ford Taurus | 22 | $0.13 |
| Nissan Altima | 23 | $0.13 |
| Honda Accord | 24 | $0.12 |
Q. How do you calculate miles per gallon of gas?
Get the miles traveled from the trip odometer, or subtract the original odometer reading from the new one. Divide the miles traveled by the amount of gallons it took to refill the tank. The result will be your car’s average miles per gallon yield for that driving period.
Q. How far will 12 gallons of gas get you?
For example, if your car averages 25 miles per gallon on the highway and has a 12-gallon fuel tank, its range is 25 x 12 = 300 miles.
Q. How much will 10 dollars of gas get you?
1 Expert Answer Since gas is $2.50/gallon and you buy $10 worth, that says you’ll get 4 gallons of gas ($2.50 * 4 = $10).
Q. What is a good amount for gas money?
Depending on the state you’re in gas prices may vary, but really the cost of the gas is minimal, like $1 probably. Time is more valuable though and at the barest minimum you should give them $5. I think $10 since it’s also wear on their car. Pay them for their time as well as their gas.
Q. How many gallons of gas is $20?
a little over 10 gallons.
Q. How many miles is a 2 hour drive?
140 miles