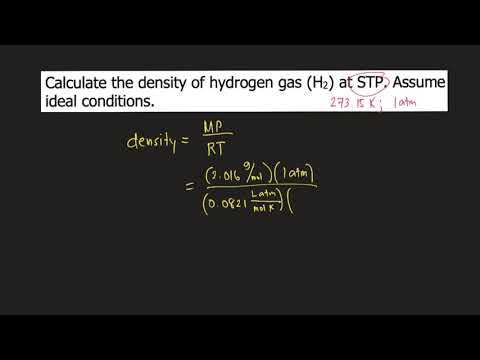
Q. How do you find the density of hydrogen?
The density of a gas is given by the equation d=PMRT d = P M R T where d is the density, P is the pressure, M is the molar mass, R is the universal gas constant and T is the temperature. STP (Standard Temperature and Pressure) condition for gases refers to a temperature of 273.15 K and a pressure of 1.0 atm.
Q. What is the density for hydrogen?
Gaseous hydrogen, with a density of 0.00523 lb/ft3, has a specific gravity of 0.0696 and is thus approximately 7% the density of air. For liquids, water (with a density of 62.4 lb/ft3; 1000 kg/m3) is used as the reference substance, so has a specific gravity of 1.0 relative to itself.
Table of Contents
- Q. How do you find the density of hydrogen?
- Q. What is the density for hydrogen?
- Q. What is the density in g/l of hydrogen gas?
- Q. What is the density of a hydrogen proton?
- Q. What is density of hydrogen at STP?
- Q. What is the density of the hydrogen gas h2 at STP?
- Q. What is the density of hydrogen in g ml?
- Q. How heavy is a hydrogen tank?
- Q. How do you calculate nuclear density?
- Q. What is protons density?
- Q. What is the maximum density of hydrogen?
- Q. How do you calculate ideal gas?
- Q. How do you calculate the ideal gas law?
- Q. What is the molar volume of hydrogen?
Q. What is the density in g/l of hydrogen gas?
The density of hydrogen gas the given conditions is 4.7 g/L.
Q. What is the density of a hydrogen proton?
The proton is not a fundamental particle, being composed of quark–gluon matter. Its size is approximately 10−15 meters and its density 1018 kg/m3.
Q. What is density of hydrogen at STP?
0.08988 g/L
| Hydrogen | |
|---|---|
| Phase at STP | gas |
| Melting point | (H2) 13.99 K (−259.16 °C, −434.49 °F) |
| Boiling point | (H2) 20.271 K (−252.879 °C, −423.182 °F) |
| Density (at STP) | 0.08988 g/L |
Q. What is the density of the hydrogen gas h2 at STP?
The density of hydrogen gas at STP is 0.09kgm−3.
Q. What is the density of hydrogen in g ml?
0.03136 g/ml
Hydrogen is a colorless, odorless gas. It is shipped in gaseous form with cylinder pressures of approximately 2000 psig @70° F….
| Molecular Weight: | 2.016 |
|---|---|
| Density, Liquid @ -253° C., 1 atm.: | 0.0708 g/l |
| Critical Temperature: | -400.° F |
| Critical Pressure: | 188.2 psia |
| Critical Density: | 0.03136 g/ml |
Q. How heavy is a hydrogen tank?
heref fore, an 8.5 gallon tank, which in a 40 mpg vehicle provides a 340-mile range (comparable to the Ford’s vehicle) weighs about 76 lb and displaces 9.0 to 9.5 gallons (34 to 36 liters).
Q. How do you calculate nuclear density?
The usual definition of nuclear density gives for its density: ρnucleus = m / V = 238 x 1.66 x 10-27 / (1.73 x 10-42) = 2.3 x 1017 kg/m3. Thus, the density of nuclear material is more than 2.1014 times greater than that of water. It is an immense density.
Q. What is protons density?
1018 kg/m3
The proton is not a fundamental particle, being composed of quark–gluon matter. Its size is approximately 10−15 meters and its density 1018 kg/m3.
Q. What is the maximum density of hydrogen?
Hydrogen has one of the highest energy density values per mass. Its energy density is between 120 and 142 MJ/kg. This means that for every 1 kg of mass of hydrogen, it has an energy value of 120-142 MJ.
Q. How do you calculate ideal gas?
Ideal gas law equation. The properties of an ideal gas are all lined in one formula of the form pV = nRT , where: p is the pressure of the gas, measured in Pa, V is the volume of the gas, measured in m^3, n is the amount of substance, measured in moles,
Q. How do you calculate the ideal gas law?
Ideal gas law equation. The properties of an ideal gas are all lined in one formula of the form pV = nRT, where: p is the pressure of the gas, measured in Pa, V is the volume of the gas, measured in m^3, n is the amount of substance, measured in moles, R is the ideal gas constant and.
Q. What is the molar volume of hydrogen?
Molar volume of the ideal hydrogen gas at room temperature (Volume/moles), expressed as L/mol at X degrees C and a pressure of 1 atmosphere = 22.4 L/mole * 0.00382 moles = 0.0856 L or 85.6mL 2. For the second part of the experiment, everything was the same except that twice as much Zn was used.