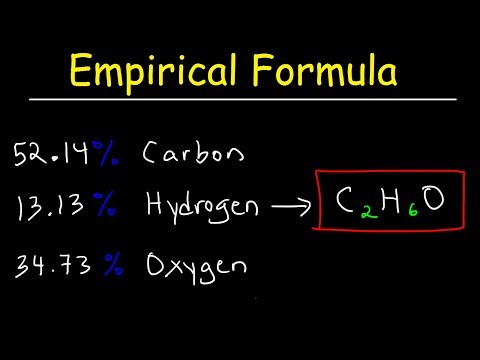
Q. How do you find the empirical formula of a substance?
Calculate the empirical formula.
- In any empirical formula problem you must first find the mass % of the elements in the compound.
- Then change the % to grams.
- Next, divide all the masses by their respective molar masses.
- Pick the smallest answer of moles and divide all figures by that.
Q. What is the empirical formula of c5h5 OH 2?
1 Answer. The empirical formula is C6H5(OH)2 .
Table of Contents
- Q. How do you find the empirical formula of a substance?
- Q. What is the empirical formula of c5h5 OH 2?
- Q. What is the empirical formula of ch3co2h?
- Q. What is the empirical formula of ethane?
- Q. What is the difference between an empirical and a molecular formula?
- Q. Which of the following compound has the same empirical formula?
- Q. What is the empirical formula of a compound containing sulfur and oxygen if the compound is 50% sulfur by mass?
- Q. What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium by weight?
- Q. Is c6h5cl an empirical formula?
- Q. What is the empirical formula of c5h12?
- Q. What is the empirical formula of benzene?
Q. What is the empirical formula of ch3co2h?
CH₃COOH
Q. What is the empirical formula of ethane?
C₂H₆
Q. What is the difference between an empirical and a molecular formula?
Empirical formulas show the simplest whole-number ratio of atoms in a compound, molecular formulas show the number of each type of atom in a molecule, and structural formulas show how the atoms in a molecule are bonded to each other.
Q. Which of the following compound has the same empirical formula?
Answer: Multiple compounds can have the same empirical formula. Glucose, C6H12O6, contains carbon, hydrogen, and oxygen. The ratio of carbon to hydrogen to oxygen is 1:2:1.
Q. What is the empirical formula of a compound containing sulfur and oxygen if the compound is 50% sulfur by mass?
5. Therefore, the empirical formula of the compound is S1O2 or simply written as SO2.
Q. What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium by weight?
Steps
| 1 | Find the empirical formula of a compound that has 50.7% of Antimony (Sb), 49.3% of Selenium (Se). |
|---|---|
| 3 | Convert to moles: Sb=0.41639290407359, Se=0.62436676798379 |
| 4 | Find smallest mole value: 0.41639290407359 |
| 5 | Divide all components my smallest value: Sb=1, Se=1.4994654372723 |
Q. Is c6h5cl an empirical formula?
C6H5Cl
Q. What is the empirical formula of c5h12?
C5H12
Q. What is the empirical formula of benzene?
C6H6
Randomly suggested related videos: