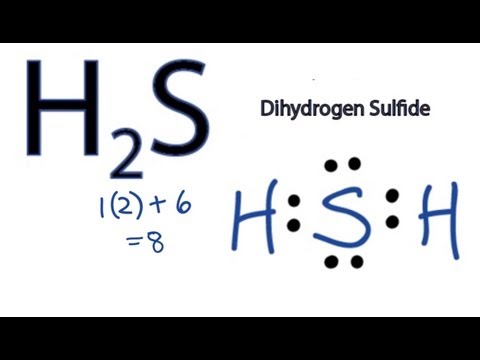
Q. How do you find the valence electrons for H2S?
To know the total number of valence electrons in Hydrogen Sulfide we need to add the valence electrons of both Hydrogen and Sulfur atoms. There are two atoms of Hydrogen and a single atom of Sulfur in the compound. Sulfur has six valence electrons. Thus, there are a total of eight valence electrons in H2S.
Q. How many atoms are in dihydrogen sulfide?
three atoms
In an H2S molecule, three atoms are present in total. Amongst the three, two are hydrogen and one of sulphur.
Table of Contents
- Q. How do you find the valence electrons for H2S?
- Q. How many atoms are in dihydrogen sulfide?
- Q. Is dihydrogen sulfide polar?
- Q. What is h2gas?
- Q. What is the formula for dihydrogen sulfide?
- Q. Is CHCL polar or nonpolar?
- Q. Is Cl2 polar or nonpolar?
- Q. Is H2 an element?
- Q. What is the PEL for H2S?
- Q. Why is dihydrogen not Monosulfide?
- Q. Is clch3 polar?
- Q. Is C2Cl4 polar or nonpolar?
- Q. How many valence electrons does H2S have?
- Q. How many valence electrons does hydrogen have on the periodic table?
- Q. What is the chemical formula for hydrogen sulfide?
- Q. What are the names of the valence electrons?
Q. Is dihydrogen sulfide polar?
H2S is a slightly polar molecule because of its bent shaped geometrical structure and the small difference between the electronegativity of Hydrogen(2.2) and Sulfur(2.58) that results in a non zero dipole moment.
Q. What is h2gas?
H2S gas is a chemical compound that stands for hydrogen sulfide carbonyl sulfide gas. It is a colorless gas and is commonly recognized by its distinct rotten egg smell. H2S gas is also widely referred to as sewer gas, sour gas, stink damp, or hydrosulphuric acid.
Q. What is the formula for dihydrogen sulfide?
H₂S
Hydrogen sulfide/Formula
Q. Is CHCL polar or nonpolar?
The electronegativity of hydrogen is 2.2, chlorine is 3.16 and that of carbon is 2.55. Therefore C-H and C-Cl bonds are polar.
Q. Is Cl2 polar or nonpolar?
Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. As a result, both atoms have equal charge distribution on them, and the molecule results in zero dipole moment that makes the chlorine molecule nonpolar.
Q. Is H2 an element?
H2, is an elemental gas with an atomic mass of 1.00794. This diatomic molecule is the lightest and most abundant element in the universe. It is also colorless, odorless, and highly flammable. Hydrogen; Hydrogen, H2, is the most abundant element in the universe.
Q. What is the PEL for H2S?
OSHA: The legal airborne permissible exposure limit (PEL) is 20 ppm not to be exceeded at any time, and 50 ppm as a maximum peak, not to be exceeded during any 10-minute work period.
Q. Why is dihydrogen not Monosulfide?
Explanation: Both are actually correct. For consistency, IUPAC has defined the “parent molecule” as “sulfane” with acceptable “official” names of both “hydrogen sulfide” and “dihydrogen sulfide” (actually preferred, both are “Compositional names” per IUPAC).
Q. Is clch3 polar?
Yes, Methyl chloride (CH3Cl) or Chloromethane is a polar molecule. The C-Cl covalent bond shows unequal electronegativity because Cl is more electronegative than carbon causing a separation in charges that results in a net dipole. What is this?
Q. Is C2Cl4 polar or nonpolar?
A NON-polar, totally symmetrical molecule like C2Cl4, known as tetrachloroethene, is used for DRY CLEANING clothes because it attracts to the non-polar grease stains that are NOT effectively removed by polar water molecules. The dry cleaning process is NOT dry at all (C2Cl4 is a liquid).
Q. How many valence electrons does H2S have?
But since you have two hydrogens you need to multiply by two. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The Lewis structure for H2S has a total of 8 valence electrons.
Q. How many valence electrons does hydrogen have on the periodic table?
On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let’s multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Total of 8 valence electrons.
Q. What is the chemical formula for hydrogen sulfide?
Hydrogen sulfide PubChem CID 402 Structure Find Similar Structures Chemical Safety Laboratory Chemical Safety Summary (LCSS Molecular Formula H2S Synonyms hydrogen sulfide sulfane Hydrosulfuric a
Q. What are the names of the valence electrons?
Element Symbol Element Name Element Element Valence Electrons 1: H: Hydrogen: 1 H – Hydrogen: 1s1 2: He: Helium: 2 He – Helium: 1s2 3: Li: Lithium: 3 Li – Lithium: 2s1 4: Be: Beryllium