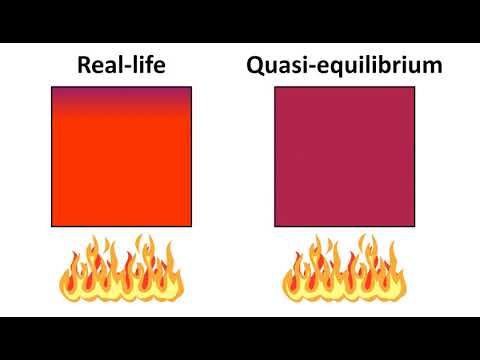
Quasi-Equilibrium Process A process during which the system only deviates from equilibrium by an infinitessimal amount. As the piston compresses the gas inside the cylinder, the pressure inside the gas remains very nearly uniform at all times during the process.
Q. What is a quasi-equilibrium process?
In thermodynamics, a quasi-static process (also known as quasi-equilibrium, from the Latin quasi, meaning ‘as if’), is a thermodynamic process that happens slowly enough for the system to remain in internal equilibrium. Any reversible process is a quasi-static one.
Table of Contents
- Q. What is a quasi-equilibrium process?
- Q. Why quasi static process is important?
- Q. What is quasi static process class 11?
- Q. What are the 4 types of thermodynamic processes?
- Q. What is Second Law of Thermodynamics class 11?
- Q. What is Second Law of Thermodynamics formula?
- Q. What is the second law of thermodynamics in simple terms?
- Q. What is the 2nd law of energy?
- Q. What does the 1st law of thermodynamics state?
- Q. Is the second law of thermodynamics always true?
- Q. Why is the 2nd Law of Thermodynamics important?
- Q. Which best describes the Second Law of Thermodynamics?
- Q. What does the second law state?
- Q. Which best describes the first law of thermodynamics?
- Q. What are the consequences of the Second Law of Thermodynamics?
- Q. How does the 2nd law of thermodynamics apply to living organisms?
- Q. Does the second law of thermodynamics apply to the universe?
- Q. What are the consequences of the first law of thermodynamics?
- Q. Is First Law of Thermodynamics important?
- Q. What is the first law of thermodynamics in simple terms?
- Q. What is the first law of efficiency?
- Q. Who proposed the first law of thermodynamics?
- Q. What is the formula to calculate work done?
- Q. How do we calculate time?
- Q. What is the formula for calculating energy efficiency?
Q. Why quasi static process is important?
Equilibrium in a system in a quasi-static process is established many times more rapidly than change in the physical parameters of the system. Quasi-static processes play an important role in thermodynamics, since thermodynamic cycles including only quasi-static processes yield maximum work values.
Q. What is quasi static process class 11?
Quasi static term means semi static . It is not purely moving. It is a hypothetical construct which means it is not in real. It is an infinitely slow process which means change from its original position is not at all significant.
Q. What are the 4 types of thermodynamic processes?
The four types of thermodynamic process are isobaric, isochoric, isothermal and adiabatic.
Q. What is Second Law of Thermodynamics class 11?
There are 2 statements of second law of thermodynamics given by two scientists: Kelvin-Planck ∫Statement: – No process is possible whose result is the absorption of heat from a reservoir and the complete conversion of the heat into work.
Q. What is Second Law of Thermodynamics formula?
The Second Law of Thermodynamics relates the heat associated with a process to the entropy change for that process. Therefore as a redox reaction proceeds there is a heat change related to the extent of the reaction, dq/dξ = T(dS/dξ).
Q. What is the second law of thermodynamics in simple terms?
The Second Law of Thermodynamics is about the quality of energy. It states that as energy is transferred or transformed, more and more of it is wasted. The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state.
Q. What is the 2nd law of energy?
Energy is the ability to bring about change or to do work. The Second Law of Thermodynamics states that “in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state.” This is also commonly referred to as entropy.
Q. What does the 1st law of thermodynamics state?
The first law of thermodynamics states that energy can neither be created nor destroyed, only altered in form.
Q. Is the second law of thermodynamics always true?
The Second Law of Thermodynamics states that entropy within an isolated system always increases. This iron-clad law has remained true for a very long time. However, researchers from the U.S. Department of Energy’s (DOE) Argonne National Laboratory may have found a way to violate this.
Q. Why is the 2nd Law of Thermodynamics important?
Why is the second law of thermodynamics so important? Second law of thermodynamics is very important because it talks about entropy and as we have discussed, ‘entropy dictates whether or not a process or a reaction is going to be spontaneous’.
Q. Which best describes the Second Law of Thermodynamics?
Which best describes the second law of thermodynamics? Energy is not created nor destroyed, but it can change into matter. Energy is not created nor destroyed, but it can change from one energy form to another.
Q. What does the second law state?
The second law states that the acceleration of an object is dependent upon two variables – the net force acting upon the object and the mass of the object. The acceleration of an object depends directly upon the net force acting upon the object, and inversely upon the mass of the object.
Q. Which best describes the first law of thermodynamics?
Which best describes the first law of thermodynamics? Energy is not created nor destroyed but it can change from one energy form to another.
Q. What are the consequences of the Second Law of Thermodynamics?
One of the most important implications of the second law is that it indicates which way time goes – time naturally flows in a way that increases disorder. The second law also predicts the end of the universe: it implies that the universe will end in a “heat death” in which everything is at the same temperature.
Q. How does the 2nd law of thermodynamics apply to living organisms?
The second law of thermodynamics states that energy can be transformed and that occurs everyday in lifeforms. As organisms take energy from their environment they can transform it into useful energy. This is the foundation of tropic dynamics.
Q. Does the second law of thermodynamics apply to the universe?
The Second Law of Thermodynamics states that the state of entropy of the entire universe, as an isolated system, will always increase over time. The second law also states that the changes in the entropy in the universe can never be negative.
Q. What are the consequences of the first law of thermodynamics?
The laws of thermodynamics are deceptively simple to state, but they are far-reaching in their consequences. The first law asserts that if heat is recognized as a form of energy, then the total energy of a system plus its surroundings is conserved; in other words, the total energy of the universe remains constant.
Q. Is First Law of Thermodynamics important?
The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The first law gives the relationship between heat transfer, work done, and the change in internal energy of a system.
Q. What is the first law of thermodynamics in simple terms?
The First Law of Thermodynamics states that heat is a form of energy, and thermodynamic processes are therefore subject to the principle of conservation of energy. This means that heat energy cannot be created or destroyed.
Q. What is the first law of efficiency?
The first law states that energy cannot be created or destroyed, but can be converted from one form. to another.
Q. Who proposed the first law of thermodynamics?
Rudolf Clausius
Q. What is the formula to calculate work done?
Work can be calculated with the equation: Work = Force × Distance. The SI unit for work is the joule (J), or Newton • meter (N • m). One joule equals the amount of work that is done when 1 N of force moves an object over a distance of 1 m.
Q. How do we calculate time?
To solve for time use the formula for time, t = d/s which means time equals distance divided by speed.
Q. What is the formula for calculating energy efficiency?
Energy efficiency is calculated by dividing the energy obtained (useful energy or energy output) by the initial energy (energy input). For example, a refrigerator has an energy efficiency of 20 to 50%, an incandescent bulb about 5%, a LED lamp over 30%, and a wind turbine 59% at most.