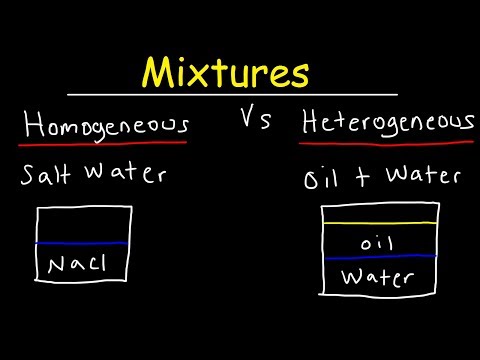
To identify the nature of a mixture, consider its sample size. If you can see more than one phase of matter or different regions in the sample, it is heterogeneous. If the composition of the mixture appears uniform no matter where you sample it, the mixture is homogeneous.
Q. What are the heterogeneous mixtures?
A heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture. A heterogeneous mixture consists of two or more phases. When oil and water are combined, they do not mix evenly, but instead form two separate layers. Each of the layers is called a phase.
Table of Contents
- Q. What are the heterogeneous mixtures?
- Q. What can you use to separate heterogeneous mixtures?
- Q. What is easier to separate a homogeneous or heterogeneous mixture?
- Q. What is the another term of homogeneous?
- Q. Is NaOH a homogeneous mixture?
- Q. Are colloids homogeneous mixtures?
- Q. Is distilled water homogeneous or heterogeneous?
Q. What can you use to separate heterogeneous mixtures?
Summary
- Mixtures can be separated using a variety of techniques.
- Chromatography involves solvent separation on a solid medium.
- Distillation takes advantage of differences in boiling points.
- Evaporation removes a liquid from a solution to leave a solid material.
- Filtration separates solids of different sizes.
Q. What is easier to separate a homogeneous or heterogeneous mixture?
Generally, I’ve found it’s easier to separate the components in a heterogeneous mixture (e.g., the pickles from hamburgers) than the components in a homogeneous mixture (e.g., the rum from a Pia Colada) because it’s easier to pick things apart when you can see the different components.
Q. What is the another term of homogeneous?
Synonyms. unvarying same undiversified homogenous homogenised self-coloured homogenized self-colored uniform consistent solid.
Q. Is NaOH a homogeneous mixture?
Sodium hydroxide NaOH is a compound. A mixture is a substance made by the physical mixing of two or more chemical substances, elements or compounds.No chemical reaction is involved.
Q. Are colloids homogeneous mixtures?
A colloid is a mixture where very small particles of one substance are evenly distributed throughout another substance. Colloids are generally considered heterogeneous mixtures, but have some qualities of homogeneous mixtures as well.
Q. Is distilled water homogeneous or heterogeneous?
Besides, is distilled water a pure substance homogeneous or heterogeneous? Distilled water is a compound , not a mixture , since it is exactly pure H 2 O .