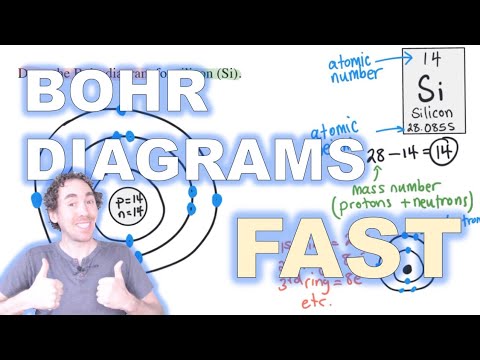
Q. How do you make a Bohr model of an element?
- Draw the nucleus.
- Write the number of neutrons and the number of protons in the nucleus.
- Draw the first energy level.
- Draw the electrons in the energy levels according to the rules below.
- Keep track of how many electrons are put in each level and the number of electrons left to use.
Q. How do you draw a Bohr Rutherford diagram?
Drawing Bohr-Rutherford diagrams is super easy using the following steps:
- Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number.
- Set up the diagram. To set up the diagram, you will need a circle in the middle.
- Add in orbitals and electrons.
Q. Which element is shown in the Bohr Rutherford model?
The Bohr atomThe Rutherford–Bohr model of the hydrogen atom. In this view, electron orbits around the nucleus resemble that of planets around the sun in the solar system.
Table of Contents
- Q. How do you make a Bohr model of an element?
- Q. How do you draw a Bohr Rutherford diagram?
- Q. Which element is shown in the Bohr Rutherford model?
- Q. Which electrons are used in the Bohr’s model?
- Q. Does it matter where the dots go on a Bohr model?
- Q. What does the line between the two letters H represent?
- Q. What is a BR diagram?
- Q. How are electrons filled in a shell?
- Q. What does Pauli’s exclusion principle say?
- Q. Where does the Pauli exclusion principle come from?
- Q. Why bosons do not follow Pauli exclusion?
- Q. What is the difference between Hund’s rule and Pauli exclusion principle?
- Q. How do you find quantum numbers?
Q. Which electrons are used in the Bohr’s model?
The Bohr model shows that the electrons in atoms are in orbits of differing energy around the nucleus (think of planets orbiting around the sun). Bohr used the term energy levels (or shells) to describe these orbits of differing energy.
Q. Does it matter where the dots go on a Bohr model?
Dots still represent electrons, but they are drawn around the symbol for the element. Only the electrons in the outermost energy level (valence electrons) are drawn. To draw an electron dot diagram, place one dot around the symbol for every valence electron.
Q. What does the line between the two letters H represent?
The hydrogen molecule, H2, consists of two hydrogen atoms sharing their two valence electrons. Each dot represents one valence electron, and the fact that they are placed between the two atoms means that they are being shared bas a covalent bond.
Q. What is a BR diagram?
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have.
Q. How are electrons filled in a shell?
Thus, the electron shells of an atom are populated from the inside out, with electrons filling up the low-energy shells closer to the nucleus before they move into the higher-energy shells further out. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons.
Q. What does Pauli’s exclusion principle say?
Pauli’s Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers. The overall magnetic activity depends upon the alignment of their unpaired electrons.
Q. Where does the Pauli exclusion principle come from?
Pauli exclusion principle, assertion that no two electrons in an atom can be at the same time in the same state or configuration, proposed (1925) by the Austrian physicist Wolfgang Pauli to account for the observed patterns of light emission from atoms.
Q. Why bosons do not follow Pauli exclusion?
Particles with an integer spin, or bosons, are not subject to the Pauli exclusion principle: any number of identical bosons can occupy the same quantum state, as with, for instance, photons produced by a laser or atoms in a Bose–Einstein condensate.
Q. What is the difference between Hund’s rule and Pauli exclusion principle?
In simple terms, the Aufbau principle means fill the orbitals from bottom to top. In simple terms, Hund’s rule requires single occupancy before pairing. Pauli Exclusion Principle. This means an orbital can hold a maximum of two electrons, and then the electrons must have opposite spins, +1/2 and -1/2.
Q. How do you find quantum numbers?
Look at the Periodic Table of Elements and find the element that you want to know the quantum number for. Find the principal number, which denotes the element’s energy, by looking in which period the element is found. For example, sodium is in the third period of the table, so its principal quantum number is 3.