Q. How do you make an ester from alcohol?
To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of concentrated sulfuric acid, and distil off the ester as soon as it is formed. This prevents the reverse reaction happening.
Q. What reaction is used to produce an ester from an alcohol and a carboxylic acid?
Fischer esterification
Table of Contents
- Q. How do you make an ester from alcohol?
- Q. What reaction is used to produce an ester from an alcohol and a carboxylic acid?
- Q. What happens when alcohol reacts with carboxylic acid?
- Q. What is ester alcohol?
- Q. How do you get rid of esters?
- Q. What are esters made of?
- Q. Is ester a salt?
- Q. How do we name Esters?
- Q. What is the name of the functional group of esters?
- Q. What is esterification give an example?
- Q. How do you test for esters?
- Q. Which process is used to determine the amount of ester in a sample?
- Q. Are esters acidic or basic?
- Q. What does ester mean in steroids?
- Q. What are the 3 types of steroids?
- Q. Do steroids have Ester?
- Q. What is the longest lasting testosterone?
- Q. What is the best testosterone to inject?
- Q. How long does a 10ml vial of testosterone last?
- Q. What’s the best testosterone to take?
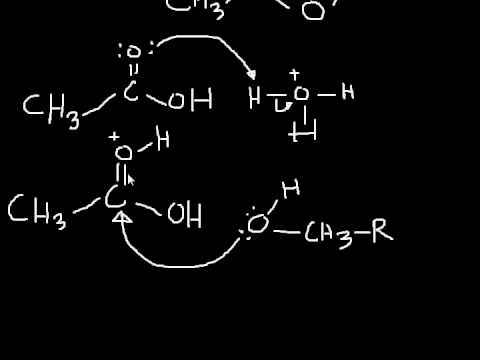
Q. What happens when alcohol reacts with carboxylic acid?
Carboxylic acids can react with alcohols to form esters in a process called Fischer esterification. An acid catalyst is required and the alcohol is also used as the reaction solvent. The oxygen atoms are color-coded in the reaction below to help understand the reaction mechanism.
Q. What is ester alcohol?
Alcohols can combine with many kinds of acids to form esters. The reaction, called Fischer esterification, is characterized by the combining of an alcohol and an acid (with acid catalysis) to yield an ester plus water. Under appropriate conditions, inorganic acids also react with alcohols to form esters.
Q. How do you get rid of esters?
Ch20: Hydrolysis of Esters. Carboxylic esters hydrolyse to the parent carboxylic acid and an alcohol. Reagents : aqueous acid (e.g. H2SO4) / heat,or aqueous NaOH / heat (known as “saponification”).
Q. What are esters made of?
An ester is a chemical compound derived from an acid (organic or inorganic) in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol.
Q. Is ester a salt?
Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming salts. The group name of the alkyl or aryl portion is given first and is followed by the name of the acid portion.
Q. How do we name Esters?
Esters are named as if the alkyl chain from the alcohol is a substituent. No number is assigned to this alkyl chain. This is followed by the name of the parent chain from the carboxylic acid part of the ester with an –e remove and replaced with the ending –oate.
Q. What is the name of the functional group of esters?
Esters are an important functional group in organic chemistry, and they are generally written RCOOR’ or RCO2R’. EstersAn ester is characterized by the orientation and bonding of the atoms shown, where R and R’ are both carbon-initiated chains of varying length, also known as alkyl groups.
Q. What is esterification give an example?
Some esters can be prepared by esterification, a reaction in which a carboxylic acid and an alcohol, heated in the presence of a mineral acid catalyst, form an ester and water: The reaction is reversible. As a specific example of an esterification reaction, butyl acetate can be made from acetic acid and 1-butanol.
Q. How do you test for esters?
One test for esters is the ferric hydroxamate test whereby the ester is converted to a hydroxamic acid (HOHN-C=O) which will give a positive ferric chloride test.
Q. Which process is used to determine the amount of ester in a sample?
Gas chromatography method has proven as the most significant method for determining the ester content till now.
Q. Are esters acidic or basic?
Esters are neutral compounds, unlike the acids from which they are formed. In typical reactions, the alkoxy (OR′) group of an ester is replaced by another group. One such reaction is hydrolysis, literally “splitting with water.” The hydrolysis of esters is catalyzed by either an acid or a base.
Q. What does ester mean in steroids?
A steroid ester is an ester of a steroid. They include androgen esters, estrogen esters, progestogen esters, and corticosteroid esters. Steroid esters may be naturally occurring/endogenous like DHEA sulfate or synthetic like estradiol valerate.
Q. What are the 3 types of steroids?
The major classes of steroid hormones, as noted above (with their prominent members and functions), are the Progestogen, Corticosteroids (corticoids), Androgens, and Estrogens.
Q. Do steroids have Ester?
Currently, almost every family of steroid hormone is known to occur in esterified form. We have studied the esters of the estrogens and glucocorticoids in some detail, and have found that these two steroidal families are esterified by separate enzymes.
Q. What is the longest lasting testosterone?
Testosterone undecanoate is a longer-acting ester that maintains serum testosterone levels within the normal range without major fluctuations and its longer half-life allows for administration every 3 months after an initial loading dose in a 6-week interval [160–162].
Q. What is the best testosterone to inject?
Testosterone Suspension. This type of testosterone contains no ester and is known among bodybuilders as a “potent mass agent.” This water-based testosterone is said to be the most powerful injectable steroid available, producing very quick muscle mass and strength.
Q. How long does a 10ml vial of testosterone last?
If a multi-dose has been opened or accessed (e.g., needle-punctured) the vial should be dated and discarded within 28 days unless the manufacturer specifies a different (shorter or longer) date for that opened vial.
Q. What’s the best testosterone to take?
Here are 8 of the best testosterone boosting supplements.
- D-Aspartic Acid. D-Aspartic acid is a natural amino acid that can boost low testosterone levels.
- Vitamin D. Vitamin D is a fat-soluble vitamin that your body produces upon exposure to sunlight.
- Tribulus Terrestris.
- Fenugreek.
- Ginger.
- DHEA.
- Zinc.
- Ashwagandha.