The gain of an electron adds more electrons to the outermost shell which increases the radius because there are now more electrons further away from the nucleus and there are more electrons to pull towards the nucleus so the pull becomes slightly weaker than of the neutral atom and causes an increase in atomic radius.
Q. Which atom is the smallest in the 5th period?
helium
Table of Contents
- Q. Which atom is the smallest in the 5th period?
- Q. Does the atomic size increase from left to right?
- Q. What increases as you move up a column of the periodic table?
- Q. What does atomic number represent?
- Q. Does removing electrons decrease radius?
- Q. What is the ionic radius of mg2+?
- Q. Does more valence electrons mean more reactive?
- Q. How do you find the inner core electrons?
- Q. What do the inner electrons do?
- Q. How many electrons are in inner shell?
- Q. What are inner electrons called?
- Q. How many core electrons does sodium have?
- Q. Why is it harder to remove an inner shell electron?
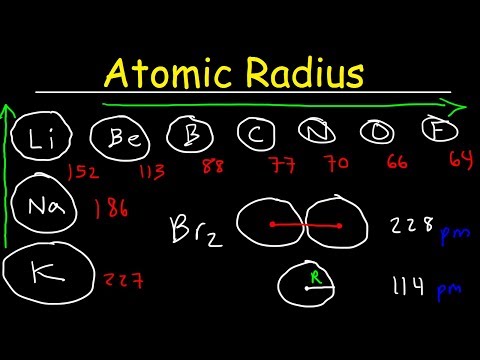
Q. Does the atomic size increase from left to right?
Atomic size gradually decreases from left to right across a period of elements. This means that the nucleus attracts the electrons more strongly, pulling the atom’s shell closer to the nucleus. The valence electrons are held closer towards the nucleus of the atom. As a result, the atomic radius decreases.
Q. What increases as you move up a column of the periodic table?
Atomic radius ( increases / decreases ) as you move from left to right across the periodic table and (increases/ decreases) as you move from top to bottom. As you move down the periodic table, you add energy levels, which makes the atoms bigger. Explain why atomic radius decreases as you move across the periodic table.
Q. What does atomic number represent?
The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).
Q. Does removing electrons decrease radius?
When an atom gains an electron, its radius increases. Conversely, when an atom loses an electron, its radius decreases.
Q. What is the ionic radius of mg2+?
Metallic, Covalent and Ionic Radii(r)*
| Atom/Ion | r(pm) |
|---|---|
| Lu3+ | 86 |
| Mg | 160 |
| Mg2+ | 72 |
| Mn | 137 |
Q. Does more valence electrons mean more reactive?
Grade 8 Atomic Structure More valence electrons adds mass to the atom, making it less reactive. Electrons have a negative charge, so when you add more valence electrons, the overall charge of the atom becomes negative, affecting its reactivity. Valence electrons don’t affect reactivity.
Q. How do you find the inner core electrons?
You can also find the core and valence electrons by determining or looking up the electron configurations of the main group elements. The atomic number is the number of protons in the nuclei of the atoms of an element. A neutral atom has the same number of electrons as protons. We can look at period 2 as an example.
Q. What do the inner electrons do?
Inner shell electrons are any electrons not in the outermost shell. They shield the valence electrons from the nucleus, reducing the effective nuclear charge.
Q. How many electrons are in inner shell?
two electrons
Q. What are inner electrons called?
Electrons in the outermost shell are called valence electrons. It is the valence electrons determine an atom’s chemical properties. Electrons in the inner shells are inner electrons or core electrons.
Q. How many core electrons does sodium have?
10 core electrons
Q. Why is it harder to remove an inner shell electron?
Explain why it is harder to remove an inner shell electron than a valence electron from an atom. Inner shell electrons are closer to the nucleus and closer to the protons, which pull the shell towards it. They try to completely fill their valence shell, so they lose the appropriate amount of electrons.