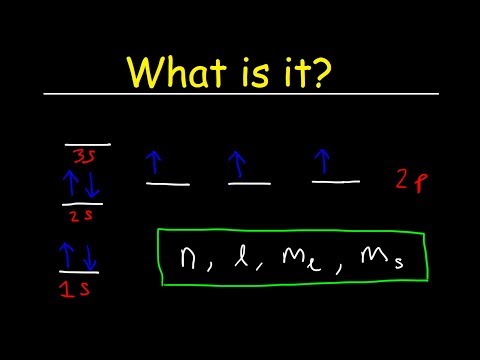
Filling in an Aufbau Diagram
Q. When two electrons have the same spin in the same orbital which principle is being violated?
Pauli’s Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers. In other words, (1) no more than two electrons can occupy the same orbital and (2) two electrons in the same orbital must have opposite spins (Figure 46(i) and (ii)).
Table of Contents
- Q. When two electrons have the same spin in the same orbital which principle is being violated?
- Q. What is Aufbau law?
- Q. Why does 3d orbital fill before 4s?
- Q. Why is 3d more energy than 4s?
- Q. Why 3d Subshell has higher energy than 4s?
- Q. How do you know which orbital is higher in energy?
- Q. Which set of orbitals can hold a maximum of 14 electrons?
- Q. What is the difference between 2s and 2p orbitals?
Q. What is Aufbau law?
The aufbau principle, from the German Aufbauprinzip (building-up principle), also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels.
- Determine the number of electrons that the atom has.
- Fill the s orbital in the first energy level (the 1s orbital) with the first two electrons.
- Fill the s orbital in the second energy level (the 2s orbital) with the second two electrons.
Q. Why does 3d orbital fill before 4s?
We say that the 4s orbitals have a lower energy than the 3d, and so the 4s orbitals are filled first. The electrons lost first will come from the highest energy level, furthest from the influence of the nucleus. So the 4s orbital must have a higher energy than the 3d orbitals.
Q. Why is 3d more energy than 4s?
According to Aufbau principle , electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only after the lower energy orbitals are filled . Therefore , 3d orbital is higher in energy than 4s . And hence electrons fill up in 4s before filling up in 3d .
Q. Why 3d Subshell has higher energy than 4s?
Ans: Once 3d orbitals are occupied by electrons, like in the case of transition elements, because they are closer to the nucleus, they will repel the 4s electrons further away from the nucleus and cause it to have higher energy level.
Q. How do you know which orbital is higher in energy?
Within a given principal energy level, electrons in p orbitals are always more energetic than those in s orbitals, those in d orbitals are always more energetic than those in p orbitals, and electrons in f orbitals are always more energetic than those in d ortitals.
Q. Which set of orbitals can hold a maximum of 14 electrons?
The d sublevel has 5 orbitals, so can contain 10 electrons max. And the 4 sublevel has 7 orbitals, so can contain 14 electrons max.
Q. What is the difference between 2s and 2p orbitals?
Notice that the 2s orbital has a slightly lower energy than the 2p orbitals. That means that the 2s orbital will fill with electrons before the 2p orbitals. All the 2p orbitals have exactly the same energy. Hydrogen only has one electron and that will go into the orbital with the lowest energy – the 1s orbital.