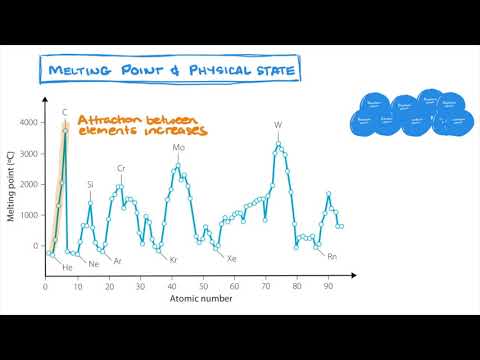
Q. How does atomic size affect melting point?
Which essentially implies breaking a few bonds. Thus, higher the stronger the bond between the atoms, higher will be the melting point.
Q. Is there a relationship between atomic number and melting point?
1. As the atomic number of elements increases, the melting point increases because there are more electrons around the nucleus, which creates a stronger negatively-charged force. With stronger forces, the melting point rises. Non-metals usually have low melting points.
Table of Contents
- Q. How does atomic size affect melting point?
- Q. Is there a relationship between atomic number and melting point?
- Q. Does atomic radius affect boiling point?
- Q. Why do smaller atoms have higher melting points?
- Q. Do bigger elements have higher melting points?
- Q. What determines melting point?
- Q. What if melting point is higher than expected?
- Q. Why melting point is different?
- Q. Why melting point is important?
- Q. What causes melting point depression?
- Q. Will snow melt at 3 degrees?
Q. Does atomic radius affect boiling point?
Atomic radius does not influence boiling point, but both are influenced by the number of electrons associated with the heavier halogens.
Q. Why do smaller atoms have higher melting points?
The atoms also get smaller and have more protons as you go from sodium to magnesium to aluminum. The attractions and therefore the melting and boiling points increase because: The nuclei of the atoms are more positively charged.
Q. Do bigger elements have higher melting points?
As you move right along the table, however, polarizability and van der Waals interactions predominate, and as larger atoms are more polarizable, they tend to exhibit stronger intermolecular forces and therefore higher melting and boiling points.
Q. What determines melting point?
But what determines a substance’s melting point? So, the melting point depends on the energy it takes to overcome the forces between the molecules, or the intermolecular forces, holding them in the lattice. The stronger the intermolecular forces are, the more energy is required, so the higher the melting point is.
Q. What if melting point is higher than expected?
Melting range broadening (the range simply increases. Often the low end drops a lot, the high end less so or sometimes not much at all.) A melting range of 5º or more indicates that a compound is impure.
Q. Why melting point is different?
The stronger the forces of attraction between the particles, the more energy is needed to overcome them. The more energy is needed, the higher the melting point. The melting temperature of a crystalline solid is thus an indicator for the stability of its lattice.
Q. Why melting point is important?
Knowing the melting point of a chemical is very important for its storage & transport. In addition to that, melting point is often used to predict the partition behavior of a chemical between solid and gas phases. A higher melting point indicates greater intermolecular forces and therefore less vapour pressure.
Q. What causes melting point depression?
Impurities in a solid cause a melting point depression because the impurity disrupts the crystal lattice energies. The more concentrated the solute, the greater the interference and the lower the freezing point of the solution. This concept can be applied to melting point (or freezing point) of a pure compound.
Q. Will snow melt at 3 degrees?
The most obvious is temperature. If the air temperature is above 32°F snow and ice will start to melt, at or below 32° and it will remain frozen. If the surface temperatures warm above 32°, the snow and ice touching the surface will warm and begin to melt.