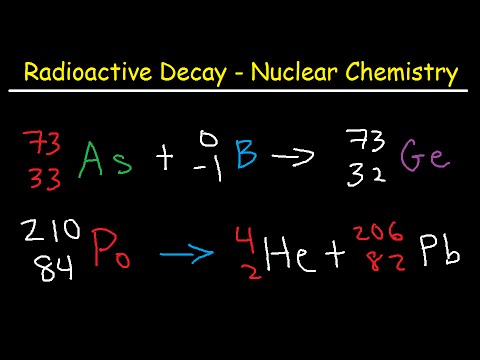
Q. How does beta decay affect mass number?
The atomic mass number does not change because a beta particle has a much smaller mass than the atom. The atomic number goes up because a neutron has turned into an extra proton. However, in beta decay, a fast moving electron is fired out of the nucleus.
Q. What happens to the mass number of an atom that undergoes beta decay?
What happens to the mass number of an element when it undergoes beta decay? Nothing. The mass number is the number of protons and neutrons.
Table of Contents
- Q. How does beta decay affect mass number?
- Q. What happens to the mass number of an atom that undergoes beta decay?
- Q. What type of particle has no mass or change?
- Q. How can you determine an atom’s mass number?
- Q. What makes up most of an atom’s mass?
- Q. Do electrons have significant mass?
- Q. Why is the mass of electrons ignored?
- Q. Where does the mass of an electron come from?
- Q. Why are electrons not included in the mass number?
- Q. What is the difference between an atom’s mass number and its atomic number?
- Q. How do you calculate the number of electrons?
- Q. What is the equation to calculate how many electrons in a shell?
- Q. What is the symbol for an electron?
- Q. How do you find the number of electrons in moles?
- Q. How many electrons are there in 10 moles?
- Q. What will be the mass and charge of one mole of electrons?
- Q. What is the mass in grams of 5 moles of Fe2O3?
- Q. What is the mass of 5 moles of?
- Q. How do you convert from moles to grams?
- Q. How many moles are in 50 grams of CO2?
Q. What type of particle has no mass or change?
In particle physics, a massless particle is an elementary particle whose invariant mass is zero. The two known massless particles are both gauge bosons: the photon (carrier of electromagnetism) and the gluon (carrier of the strong force).
Q. How can you determine an atom’s mass number?
Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
Q. What makes up most of an atom’s mass?
The majority of an atoms’ mass comes from the protons and neutrons that make up its nucleus.
Q. Do electrons have significant mass?
An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom. The electron was discovered in 1897 by the English physicist J.J.
Q. Why is the mass of electrons ignored?
Atomic mass is a measure of how massive an atom is (measured in special units called atomic mass units) it is determined by adding the number of protons and neutrons (we can do this because protons and neutrons have about the same mass, and we ignore the electrons because their mass is much much smaller than either a …
Q. Where does the mass of an electron come from?
All of the atom’s mass comes from its nucleus, but all of its size comes from the fact that the electron refuses to get too close to the proton. Most of the atom is empty space. Vacuum.
Q. Why are electrons not included in the mass number?
1 Expert Answer The mass of an electron is so small compared to the mass of a proton and a neutron, that it is generally neglected when calculating the atomic mass of an element. A proton has a mass of about 1800 times that of an electron.
Q. What is the difference between an atom’s mass number and its atomic number?
The major difference between atomic number and mass number is that the atomic number states the number of protons present in an atom whereas, the mass number indicates the total of the number of protons and the number neutrons present in an atom.
Q. How do you calculate the number of electrons?
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
Q. What is the equation to calculate how many electrons in a shell?
Rule 1: The maximum number of electrons present in a particular shell is calculated by the formula 2n2, where “n” represents the shell number. For instance, K shell is the first shell and it can hold up to 2(1)2 = 2 electrons. Similarly, L shell is the second shell and it can hold up to 2(2)2 = 8 electrons.
Q. What is the symbol for an electron?
e −
Q. How do you find the number of electrons in moles?
moles of electrons = coulombs / F = 6.673 x 102 / 96487 = 6.916 x 10-3 mol. from which, one mole of H2 formed requires two moles of electrons.
Q. How many electrons are there in 10 moles?
An atom of hydrogen has 1 electron, an atom of oxygen has 8 electrons. Thus, in one molecule of water there are 10 electrons. So, in one mole of water there are 10 moles of electrons. And so, in 10 moles of water there are 100 moles of electrons.
Q. What will be the mass and charge of one mole of electrons?
Now, we know the number of electrons present in 1 mole of electrons and the mass and charge of an electron. Thus, the mass of one mole of electrons is 5. 48×10 – 7kg. Thus, the charge of one mole of electrons is 9.
Q. What is the mass in grams of 5 moles of Fe2O3?
What is the mass of 5 moles of Fe2O3? 1st find Molar Mass of fe 02 Fe = (55.85)/2) = 111.7g Total = 159.78] Smol l 159.79 rezoz – 799 Garans Poon BO=(16Y3) – 489 Timol = 798.5 3. Find the number of moles of argon in 452 g of argon.
Q. What is the mass of 5 moles of?
So2 = 32 + 32 = 64 grams. = 320 grams. Therefore, the mass of 5 moles of So2 is approximately equal to 320 grams.
Q. How do you convert from moles to grams?
You have three steps to convert mole values to grams.
- Calculate how many moles are mentioned in the question.
- Find the molar mass of the substance.
- Multiply both the values.
Q. How many moles are in 50 grams of CO2?
Explanation: The answer is 44.0095. We assume you are converting between grams CO2 and mole.