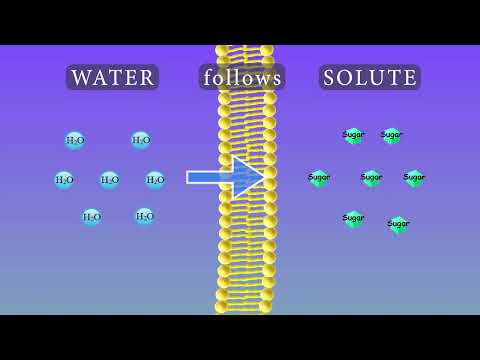
Q. How does concentration affect osmosis?
Concentration gradient – The movement of osmosis is affected by the concentration gradient; the lower the concentration of the solute within a solvent, the faster osmosis will occur in that solvent. Light and dark – They are also factors of osmosis; since the brighter the light, the faster osmosis takes place.
Q. How are concentration and diffusion related?
1 Answer. Molecules diffuse from areas of high concentration, to areas of low concentration, down a concentration gradient. Higher concentration gradients will result in higher rates of diffusion. As the molecules move the gradient evens out until equilibrium is reached.
Table of Contents
- Q. How does concentration affect osmosis?
- Q. How are concentration and diffusion related?
- Q. How does solute concentration affect diffusion osmosis?
- Q. How does concentration gradient affect diffusion and osmosis?
- Q. Which best describes the difference between osmosis and diffusion?
- Q. What are the 5 factors that affect diffusion?
- Q. What is the formula for rate of diffusion?
- Q. How does size affect rate of diffusion?
- Q. Does pH affect rate of diffusion?
- Q. What are the factors that affect diffusion?
- Q. What does not affect diffusion?
- Q. How do you find the highest rate of diffusion?
- Q. Which of the following has the highest rate of diffusion?
- Q. Which state of matter has higher rate of diffusion?
- Q. Which substance has the highest diffusion rate?
- Q. Which gas has the lowest rate of diffusion?
- Q. What is another example of diffusion?
- Q. At what temperature is a gas likely to have the lowest rate of diffusion 15 21 24 30?
- Q. Which gas is likely to have the lowest rate of diffusion 4 points?
- Q. Why rate of diffusion is faster in gases?
- Q. What gas diffuses the fastest?
- Q. Which diffuses faster liquid or gas?
- Q. At what temperature will gas molecule in a room be moving the slowest?
- Q. Which matter diffuses the fastest?
- Q. What is the fastest to slowest rate of diffusion?
- Q. Which state of matter has no definite shape?
- Q. What state of matter is the rate of diffusion fastest slowest?
- Q. What are the 22 states of matter?
- Q. Which state of matter has the highest rate of diffusion and why?
- Q. Which state of matter has the lowest density?
Q. How does solute concentration affect diffusion osmosis?
Osmosis occurs according to the concentration gradient of water across the membrane, which is inversely proportional to the concentration of solutes. Osmosis occurs when there is a concentration gradient of a solute within a solution, but the membrane does not allow diffusion of the solute.
Q. How does concentration gradient affect diffusion and osmosis?
In other words, diffusion occurs down the concentration gradient of the molecule in question. If the difference in concentration is higher, then the molecules will go down the concentration gradient faster.
Q. Which best describes the difference between osmosis and diffusion?
In diffusion, particles move from an area of higher concentration to one of lower concentration until equilibrium is reached. In osmosis, a semipermeable membrane is present, so only the solvent molecules are free to move to equalize concentration.
Q. What are the 5 factors that affect diffusion?
Several factors affect the rate of diffusion of a solute including the mass of the solute, the temperature of the environment, the solvent density, and the distance traveled.
Q. What is the formula for rate of diffusion?
Graham’s Law Formula Graham’s law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. See this law in equation form below. In these equations, r = rate of diffusion or effusion and M = molar mass.
Q. How does size affect rate of diffusion?
When the cell increases in size, the volume increases faster than the surface area, because volume is cubed where surface area is squared. When there is more volume and less surface area, diffusion takes longer and is less effective. this is actually why cells divide.
Q. Does pH affect rate of diffusion?
Why does pH affect the rate of diffusion? The acidity of alkaline properties of the solute can affect the stability of the cell(s). The ability of the solute being able to diffuse in each state depending on the state the substance is in as well. Solid diffusing into a liquid.
Q. What are the factors that affect diffusion?
The rate of diffusion
| Factor | Reason |
|---|---|
| The temperature | The higher the temperature, the more kinetic energy the particles will have, so they will move and mix more quickly. |
| The surface area of the cell membrane separating the different regions | The greater the surface area, the faster the rate of diffusion. |
Q. What does not affect diffusion?
The factor that does not affect the rate of diffusion are the electrical charges of the diffusion particles.
Q. How do you find the highest rate of diffusion?
As rate of diffusion is inversely proportional to square root of molar mass, Gas with lowest molar mass will have highest rate of diffusion..
Q. Which of the following has the highest rate of diffusion?
NH3 has lesser molecular mass than the above gases, so the rate of diffusion of NH3 is higher. This is according to the Graham’s law of Diffusion of gases.
Q. Which state of matter has higher rate of diffusion?
Hence we can conclude the rate of diffusion of liquids is higher than solids due to the free movement of molecules and lack of strong force of attraction in liquids but rate of diffusion of liquids is lower than gases as in gases molecules are quite far apart.
Q. Which substance has the highest diffusion rate?
This is an important factor affecting the difference in diffusion rates in gases versus liquids versus solids; because gas particles are the most spread out of the three, molecules travel the furthest between collisions and diffusion occurs most rapidly in this state (Figure 5).
Q. Which gas has the lowest rate of diffusion?
As per Graham’s law, the rate of effusion or diffusion is inversely proportional to the square root of its molar mass. Hence, the gas with the highest molecular weight diffuses the slowest. Hence, Chlorine is the slowest when it comes to rate of diffusion and helium is the fastest as it is the lightest.
Q. What is another example of diffusion?
Perfume is sprayed in one part of a room, yet soon it diffuses so that you can smell it everywhere. A drop of food coloring diffuses throughout the water in a glass so that, eventually, the entire glass will be colored.
Q. At what temperature is a gas likely to have the lowest rate of diffusion 15 21 24 30?
15º
Q. Which gas is likely to have the lowest rate of diffusion 4 points?
F2 has lowest rate of diffusion.
Q. Why rate of diffusion is faster in gases?
Diffusion in gases is quick because the particles in a gas move quickly. It happens even faster in hot gases because the particles of gas move faster.
Q. What gas diffuses the fastest?
The rate of effusion for a gas is inversely proportional to the square-root of its molecular mass (Graham’s Law). The gas with the lowest molecular weight will effuse the fastest. The lightest, and therefore fastest, gas is helium.
Q. Which diffuses faster liquid or gas?
Gas diffuses faster than liquid because the particles of gases are more spacious and have high kinetic energy.
Q. At what temperature will gas molecule in a room be moving the slowest?
At what temperature will gas molecules in a room be moving the slowest? a. 15°Cb.
Q. Which matter diffuses the fastest?
gases
Q. What is the fastest to slowest rate of diffusion?
Neon is the fastest. Chlorine is the slowest.
Q. Which state of matter has no definite shape?
gas
Q. What state of matter is the rate of diffusion fastest slowest?
Diffusion is the property of matter which is based on the motion of its particles. Diffusion occurs in gases, liquids and solids. Diffusion is fastest in gases and slowest in solids.
Q. What are the 22 states of matter?
- Bose–Einstein condensate.
- Fermionic condensate.
- Degenerate matter.
- Quantum Hall.
- Rydberg matter.
- Rydberg polaron.
- Strange matter.
- Superfluid.
Q. Which state of matter has the highest rate of diffusion and why?
Gaseous particles are in constant random motion. Gaseous particles tend to undergo diffusion because they have kinetic energy. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy.