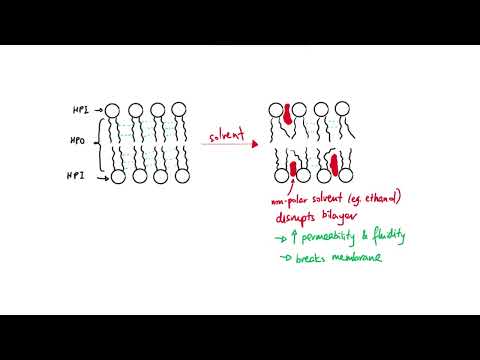
Q. How does ethanol affect membrane permeability?
At high concentrations, alcohols reduce bilayer stability (12, 21) and break down the lipid bilayer barrier properties, causing increased ion permeability (14, 15).
Q. Can ethanol pass through cell membrane?
Small polar molecules, such as water and ethanol, can also pass through membranes, but they do so more slowly. On the other hand, cell membranes restrict diffusion of highly charged molecules, such as ions, and large molecules, such as sugars and amino acids.
Q. How does ethanol affect cell membrane?
Ethanol disrupts the physical structure of cell membranes. When animals are treated chronically with ethanol, their membranes become stiffer, a response that can be regarded as adaptive. Ethanol may favor the uptake of cholesterol or saturated fatty acids into membranes, thus reducing its own effect.
Q. Why does ethanol make membranes more permeable?
Ethanol is able to form hydrogen bonds with the lipids in the bilayer (see Hydrogen Bonding of Alcohol to Lipids, below), and these hydrogen bonds reduce the order parameter of the lipid hydrocarbon chains. The combination of these aspects results in an easy penetration of ethanol through the bilayer.
Q. What happens to membrane permeability below 0?
Generally, increasing the temperature increases membrane permeability. At temperatures below 0 oC the phospholipids in the membrane don’t have much energy and so they can’t move much, which means that they’re closely packed together and the membrane is rigid.
Q. What does ethanol do to the membrane lipid bilayer?
At high concentrations, alcohols reduce bilayer stability (12,21) and break down the lipid bilayer barrier properties, causing increased ion permeability (14,15).
Q. Can ethanol pass through lipid bilayer?
Ethanol can also diffuse through the core of the lipid bilayer, this is because it is also slightly lipophilic (lipid loving) and relatively small. Other kinds of polar compounds are charged, which prevents them from diffusing through the hydrophobic core of the membrane (they can only dissolve in water).
Q. Is ethanol toxic to cells?
Conclusion: Ethanol seemed to kill cells in the cell culture effectively in much lower concentrations than those currently used in tumour ablation.
Q. Does pH affect membrane permeability?
The pH of the solution that the beetroot is placed in has a large effect on the permeability of the cell membrane. This is because like changes in temperature, pH values that are not optimal for the protein will denature it causing it to not function and, in this case, allow betacyanin to leak through.
Q. Why does solvent affect membrane permeability?
Going from a good solvent for PA (water) to poorer solvents (increasing alcohol concentration), the permeability increased. For example, the hydrodynamic permeability of the membrane-supported gels increased 100 times at alcohol concentrations for which bulk gel collapsed by a factor of 7 in volume.
Q. What affects membrane permeability?
In this article, it is shown that membrane permeability to water and solutes is dependent on the temperature, medium osmolality, types of solutes present, cell hydration level, and absence or presence of ice.
Q. What effect might a solvent have on membrane permeability?
Ethanol is a non-polar solvent so it is able to dissolve non-polar substances such as lipids. This means that if you place a cell in ethanol, its membrane will become permeable and allow substances to leak into and out of the cell. As the ethanol concentration increases, membrane permeability will increase.
Q. What affects membrane structure and permeability?
Q. How does temperature affect beetroot cell membranes?
In beetroot cells, along with water and other molecules, the vacuole contains a pigment called betalain. When the conditions become warmer, the cell membrane is disrupted, causing the vacuole to release greater amounts of betalain through the more permeable membrane.
Q. Why does temperature increase membrane permeability?
The higher the temperature, the greater the kinetic energy and the faster the movement and diffusion of pigment molecules. Greater kinetic energy also causes phospholipids of the membrane to become more fluid and bonds between the fatty acid tails can begin to separate so that some pigment molecules can pass through.
Q. What is the optimal temperature for beet cell membranes?
The 25% solution was made of 10 mL methylated spirits and 30 mL distilled water; the 50% solution used 20 mL methylated spirits and 20 mL distilled water….Procedure.
| Temperature | Colour intensity – from 1 (light) to 5 (dark) | Star Scale – from 1 (light) to 5(dark) |
|---|---|---|
| 70°C | 2 | ** |
| 80°C | 4 | **** |
Q. What would increase membrane fluidity?
Membrane fluidity can be affected by a number of factors. One way to increase membrane fluidity is to heat up the membrane. Lipids acquire thermal energy when they are heated up; energetic lipids move around more, arranging and rearranging randomly, making the membrane more fluid.
Q. Why is fluidity important in membrane structure?
Fluidity is important for many reasons: 1. it allows membrane proteins rapidly in the plane of bilayer. 2. It permits membrane lipids and proteins to diffuse from sites where they are inserted into bilayer after their synthesis.
Q. What factors increase membrane fluidity?
The ratio of saturated and unsaturated fatty acids determines the fluidity in the membrane at cold temperatures. Cholesterol functions as a buffer, preventing lower temperatures from inhibiting fluidity and preventing higher temperatures from increasing fluidity.
Q. Why does cholesterol decrease membrane fluidity?
By decreasing the mobility of a few methylene groups (CH2) in the fatty acids tails, cholesterol makes lipid bilayers less deformable and lessens their permeability to small water-soluble molecules. Therefore, cholesterol makes membranes less fluid.
Q. What lipid makes the membrane more fluid?
In addition to phospholipids, animals have an additional membrane component that helps to maintain fluidity. Cholesterol, another type of lipid that is embedded among the phospholipids of the membrane, helps to minimize the effects of temperature on fluidity.
Q. Which type of lipid is most important in biological membranes?
The most abundant membrane lipids are the phospholipids. These have a polar head group and two hydrophobic hydrocarbon tails.
Q. Which is the most important in biological membranes?
Membrane proteins play a vital role in biological membranes, as they help to maintain the structural integrity, organization and flow of material through membranes. Sugars are found on one side of the bilayer only, and are attached by covalent bonds to some lipids and proteins.
Q. Which type of lipid is most important in biological membranes quizlet?
The most abundant lipid in most membranes are phospholipids. The ability of phospholipids to form membranes is inherent in their molecular structure. A phospholipid is an amphipathic molecule, meaning that it has both a hydrophilic region and a hydrophobic region.
Q. What is the most important component in biological membranes?
The main components of biological membranes are proteins, lipids, and carbohydrates in variable proportions. Carbohydrates account for less than 10% of the mass of most membranes and are generally bound either to the lipid or protein components. Myelin has few functions and is made up almost entirely of lipids.
Q. Are there triacylglycerols in membranes?
Recent observations have confirmed triacylglycerol (TG) as a quantitatively minor intrinsic membrane component which seems to play a specific role in important metabolic events such as cell stimulation or transformation and metastatic processes.
Q. Why are triacylglycerols not found in cell membranes?
They contain three chains of fatty acids. Although similar in structure to the phospholipids that build cell membranes, triglycerides are completely hydrophobic, meaning they cannot mix with water, so they cannot integrate into membranes.
Q. Where are new membranes?
In eucaryotic cells, new phospholipids are manufactured by enzymes bound to the part of the endoplasmic reticulum membrane that faces the cytosol. These enzymes, which use free fatty acids as substrates, deposit all newly made phospholipids into the cytosolic half of the bilayer.
Lipids containing unsaturated fatty acids similarly increase membrane fluidity because the presence of double bonds introduces kinks in the fatty acid chains, making them more difficult to pack together.