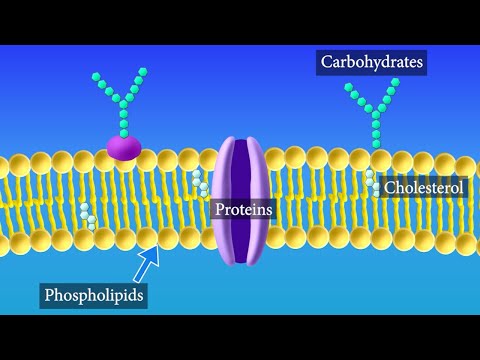
Q. How does hydrophobic relate to the structure of the cell membrane?
Phospholipids, arranged in a bilayer, make up the basic fabric of the plasma membrane. The hydrophobic, or “water-fearing,” part of a phospholipid consists of its long, nonpolar fatty acid tails. The fatty acid tails can easily interact with other nonpolar molecules, but they interact poorly with water.
Q. How is hydrophilic and hydrophobic important to cell membranes?
Hydrophobic and Hydrophilic Hydrocarbon molecules are strongly hydrophobic (“water fearing”), and it is this strongly hydrophobic layer of material that gives the cell membrane its “water proof” nature and allows it to act as a container for the cell and its contents.
Table of Contents
- Q. How does hydrophobic relate to the structure of the cell membrane?
- Q. How is hydrophilic and hydrophobic important to cell membranes?
- Q. What do these terms hydrophilic and hydrophobic mean and how do they relate to the structure of a cell membrane quizlet?
- Q. How do the hydrophilic and hydrophobic properties of the phospholipids help to maintain the structure of the cell membranes?
- Q. What has hydrophobic and hydrophilic properties?
- Q. What are examples of hydrophilic?
- Q. Do hydrophobic and hydrophilic attract?
- Q. What makes a structure hydrophilic?
- Q. What is the different in the structure and function of hydrophobic and hydrophilic side chains?
- Q. What drives the hydrophobic effect?
- Q. What is the hydrophobic effect simple terms?
- Q. What is the function of hydrophobic?
- Q. How do hydrophobic interactions work?
- Q. What is an example of a hydrophobic molecule?
- Q. Does pH affect hydrophobic interactions?
- Q. Where do hydrophobic interactions occur?
- Q. How do you know if a molecule is hydrophobic?
- Q. How can you prevent hydrophobic interactions?
- Q. What is the difference between van der Waals and hydrophobic interactions?
- Q. What are the three types of van der Waals forces?
- Q. What is the strongest noncovalent interaction?
- Q. What does the word hydrophobic mean?
- Q. What is hydrophobic explain and give examples?
- Q. Is hydrophobic a real word?
- Q. What is another word for hydrophobic?
- Q. How do you use hydrophobic in a sentence?
- Q. What’s the opposite of hydrophobic?
- Q. What is hydrophilic and hydrophobic?
Q. What do these terms hydrophilic and hydrophobic mean and how do they relate to the structure of a cell membrane quizlet?
What do these terms mean? The phosphate group and polar head region is hydrophilic the fatty acids are hydrophobic. Hydrophilic means attracted to water and hydrophobic means repelled by water.
Q. How do the hydrophilic and hydrophobic properties of the phospholipids help to maintain the structure of the cell membranes?
Due to the interactions between the hydrophobic tails in the centre and between the hydrophilic heads and water on the outside, it creates a very stable membrane.
Q. What has hydrophobic and hydrophilic properties?
Hydrophobic materials repel water, while hydrophilic materials attract or absorb water. Materials like lotus leaves, magic sand and nano-tex fabric are all examples of hydrophobic materials. Materials like sodium polyacrylate (found in diapers) and instant snow are hydrophilic materials.
Q. What are examples of hydrophilic?
The degree or extent to which a molecule or surface attracts water is known as the ‘hydrophilicity’ of that molecule. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose.
Q. Do hydrophobic and hydrophilic attract?
In summary, most hydrophilic polymers have a high positive Δ G iwi value, so that they strongly repel one another, but are nonetheless strongly attracted, in water, to significantly hydrophobic surfaces.
Q. What makes a structure hydrophilic?
A hydrophilic molecule or portion of a molecule is one whose interactions with water and other polar substances are more thermodynamically favorable than their interactions with oil or other hydrophobic solvents. They are typically charge-polarized and capable of hydrogen bonding.
Q. What is the different in the structure and function of hydrophobic and hydrophilic side chains?
The key difference between hydrophobic and hydrophilic amino acids is that the hydrophobic amino acids are nonpolar whereas the hydrophilic amino acids are polar. Amino acids are the building blocks of proteins. A protein is a giant polymer molecule which is an essential component of all living organisms.
Q. What drives the hydrophobic effect?
The hydrophobic effect is caused by the exclusion of nonpolar moieties from an aqueous environment and which drives the aggregation of these nonpolar solutes.
Q. What is the hydrophobic effect simple terms?
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity.
Q. What is the function of hydrophobic?
Hydrophobic literally means “the fear of water”. Hydrophobic molecules and surfaces repel water. Hydrophobic liquids, such as oil, will separate from water. Hydrophobic molecules are usually nonpolar, meaning the atoms that make the molecule do not produce a static electric field.
Q. How do hydrophobic interactions work?
Hydrophobic interactions describe the relations between water and hydrophobes (low water-soluble molecules). Hydrophobes are nonpolar molecules and usually have a long chain of carbons that do not interact with water molecules. The mixing of fat and water is a good example of this particular interaction.
Q. What is an example of a hydrophobic molecule?
Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds.
Q. Does pH affect hydrophobic interactions?
The effect of pH on hydrophobic interactions was not underestimated. Indeed, clear reduction of the surface hydrophobicity of proteins at low pH has already been described [19] and higher retentions of proteins on hydrophobic sorbents have been observed at high pH [20] .
Q. Where do hydrophobic interactions occur?
Hydrophobic interactions occur in amphipathic molecules in order to stop the water molecules’ ability to surround them, the hydrophillic regions are orientated in a way that allows them to act as a protective outer structure because it interacts amicably with water.
Q. How do you know if a molecule is hydrophobic?
If all the bonds in a molecule are nonpolar, then the molecule itself is nonpolar. Some examples of nonpolar covalent bonds are C-C and C-H bonds. 2. Even if a molecule has polar covalent bonds, if these bonds are arranged symmetrically, the molecule overall will be hydrophobic.
Q. How can you prevent hydrophobic interactions?
Organic solvents commonly used to weaken, or disrupt hydrophobic interactions include glycols, acetonitrile and alcohols. The organic solvents alter the polarity of the mobile phase, thereby weakening potential interactions that may occur.
Q. What is the difference between van der Waals and hydrophobic interactions?
The key difference between Van der Waals and hydrophobic interactions is that Van der Waals interactions are attraction forces between non-polar molecules, whereas hydrophobic interactions are repulsion forces between water molecules and other molecules.
Q. What are the three types of van der Waals forces?
The three types of van der Waals forces include: 1) dispersion (weak), 2) dipole-dipole (medium), and 3) hydrogen (strong). Ion-dipole bonds (ionic species to covalent molecules) are formed between ions and polar molecules. These compounds typically form medium to strong bonds.
Q. What is the strongest noncovalent interaction?
The strongest type of non-covalent interaction is between two ionic groups of opposite charge (an ion-ion or charge-charge interaction).
Q. What does the word hydrophobic mean?
Biology Glossary search by EverythingBio.com. Meaning ” water fearing”. Hydrophobic compounds do not dissolve easily in water, and are usually non-polar.
Q. What is hydrophobic explain and give examples?
Hydrophobic (biology definition): lacking an affinity for water; insoluble in water; repelling water. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general.
Q. Is hydrophobic a real word?
adj. Repelling, tending not to combine with, or unable to dissolve in water. Of or exhibiting hydrophobia.
Q. What is another word for hydrophobic?
In this page you can discover 15 synonyms, antonyms, idiomatic expressions, and related words for hydrophobic, like: aquaphobic, hydrophilic, , side-chain, hydrogen-bonding, dimer, chromophore, in solution, nonpolar, ligand and zwitterionic.
Q. How do you use hydrophobic in a sentence?
Hydrophobic sentence example
- Hydrophobic : The Hydrophobic technology actually repels dirt and dust, all while remaining impenetrable to oil and sweat.
- Hydrophobic and anti-reflective coatings are layered on each lens in order to give you the least amount of glare possible.
Q. What’s the opposite of hydrophobic?
The opposite of hydrophobic is hydrophilic, water-loving. Surface-active agents contain both hydrophobic and hydrophilic groups on the same molecules.
Q. What is hydrophilic and hydrophobic?
Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Those that naturally repel water, causing droplets to form, are known as hydrophobic.