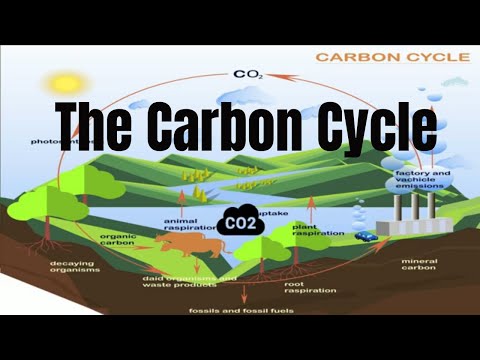
Q. How does the carbon cycle demonstrate the law of conservation of matter?
The Law of Conservation of Matter states that matter cannot be created or destroyed. The carbon cycle is an example of the Law because the same carbon atoms are being recycled through the carbon cycle. Carbon is used for energy, and some is stored for growth.
Q. How do you demonstrate the law of conservation of mass?
The Law of Conservation of Mass states that substances involved in chemical reactions do not lose or gain any detectable mass. The state of the substance, however, can change. For instance, the Law of Conservation of Mass should prove that an ice cube will have the same mass as the water that forms as the cube melts.
Table of Contents
- Q. How does the carbon cycle demonstrate the law of conservation of matter?
- Q. How do you demonstrate the law of conservation of mass?
- Q. How is carbon conserved throughout the carbon cycle?
- Q. How does the nitrogen cycle demonstrate the law of conservation of matter?
- Q. Is the nitrogen cycle a closed system?
- Q. What do you mean by Law of Conservation of Mass?
- Q. Which one is the best example of law of conservation of mass?
- Q. What is the law of conservation of matter explain with example?
- Q. What is the law of conservation of mass Class 9?
- Q. What is atomicity Class 9?
- Q. How is the law of conservation of mass used in everyday life?
- Q. What is law of constant proportion class 9th?
- Q. Who proposed law of constant proportion?
- Q. What atomicity means?
- Q. What is law of conservation of mass and law of constant proportions Class 9?
- Q. What is the law of conservation of matter and energy?
- Q. What is the meaning of molecule?
- Q. What do you mean by law of definite proportion?
- Q. What is the law of definite proportion explain with an example?
- Q. Is the law of definite proportions true?
- Q. What is the difference between the law of conservation of mass and the law of definite proportion?
- Q. Which atom is used today as the standard for?
- Q. Can atoms be created or destroyed?
- Q. Why is there a need to attain the law of conservation of mass?
- Q. What are the chemical reaction according to the law of conservation of mass?
- Q. Why is it hard to prove the law of conservation of mass when a gas is produced?
- Q. What are the limitations of the law of conservation of mass?
- Q. Is the law of conservation of mass always true?
- Q. Does the law of conservation of mass apply to every reaction?
- Q. Can mass be destroyed?
Q. How is carbon conserved throughout the carbon cycle?
Carbon that is used by producers, consumers and decomposers cycles fairly rapidly through air, water and biota. But carbon can also be stored as biomass in the roots of trees and other organic matter for many decades. This carbon is released back into the atmosphere by decomposition, as was noted before.
Q. How does the nitrogen cycle demonstrate the law of conservation of matter?
During the nitrogen cycle, nitrogen atoms are neither created or destroyed, or changed into other atoms. This can be explained using the law of conservation of matter, which states that matter is never created or destroyed in any chemical or physical proess.
Q. Is the nitrogen cycle a closed system?
Matter on the earth operates in a closed system where the atoms and molecules continually cycle around through the earth’s systems. This is the case for both the carbon cycle and the nitrogen cycle. Nitrogen is mainly found in the atmosphere as well and enters the ecosystems as nutrients for plants.
Q. What do you mean by Law of Conservation of Mass?
The Law of Conservation of Mass dates from Antoine Lavoisier’s 1789 discovery that mass is neither created nor destroyed in chemical reactions. In other words, the mass of any one element at the beginning of a reaction will equal the mass of that element at the end of the reaction.
Q. Which one is the best example of law of conservation of mass?
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases equals the original mass of the charcoal and the oxygen when it first reacted.
Q. What is the law of conservation of matter explain with example?
The Law of Conservation of Mass Matter can change form through physical and chemical changes, but through any of these changes, matter is conserved. The same amount of matter exists before and after the change—none is created or destroyed. This concept is called the Law of Conservation of Mass.
Q. What is the law of conservation of mass Class 9?
Laws of conservation of mass. Laws of conservation of mass. The law states that mass can neither be created nor destroyed in a chemical reaction i.e. Total masses of reactants is equal to the sum of masses of products and the masses of unreacted reactants.
Q. What is atomicity Class 9?
Class 9 Chemistry Atoms and Molecules. Atomicity. Atomicity. The number of atoms present in a single molecule is termed as its atomicity.
Q. How is the law of conservation of mass used in everyday life?
Q. What is law of constant proportion class 9th?
The law of constant proportions states that chemical compounds are made up of elements that are present in a fixed ratio by mass. This implies that any pure sample of a compound, no matter the source, will always consist of the same elements that are present in the same ratio by mass.
Q. Who proposed law of constant proportion?
Joseph Proust
Q. What atomicity means?
Atomicity is defined as the total number of atoms present in a molecule. For example, each molecule of oxygen (O2) is composed of two oxygen atoms. So atomicity of oxygen is 2.In older contexts, the term atomicity is sometimes used in the same sense as valency.
Q. What is law of conservation of mass and law of constant proportions Class 9?
Laws of conservation of mass – It states that mass can neither created nor destroyed. The total mass before and after a chemical reaction remains constant. Laws of constant proportion – It states that in a chemical substance the elements are always present in a fixed proportion by their mass.
Q. What is the law of conservation of matter and energy?
The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed. The carbon atom changes from a solid structure to a gas but its mass does not change. Similarly, the law of conservation of energy states that the amount of energy is neither created nor destroyed.
Q. What is the meaning of molecule?
Molecule, a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance. Several methods of representing a molecule’s structure.
Q. What do you mean by law of definite proportion?
Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent elements.
Q. What is the law of definite proportion explain with an example?
Common Examples of the Law of Definite Proportions. Water, written as the chemical compound H20, is made up of atoms of hydrogen and oxygen. If one oxygen atom is combined with two hydrogen atoms, water is created.
Q. Is the law of definite proportions true?
Although very useful in the foundation of modern chemistry, the law of definite proportions is not universally true. There exist non-stoichiometric compounds whose elemental composition can vary from sample to sample. Such compounds follow the law of multiple proportion.
Q. What is the difference between the law of conservation of mass and the law of definite proportion?
The mass of matter is always the same before and after the changes occur. The law of conservation of mass states that matter cannot be created or destroyed. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass.
Q. Which atom is used today as the standard for?
Explanation: The carbon-12 atom, which is still used as the standard today, contains six protons and six neutrons for an atomic mass of twelve amu.
Q. Can atoms be created or destroyed?
All matter consists of indivisible particles called atoms. Atoms of the same element are similar in shape and mass, but differ from the atoms of other elements. Atoms cannot be created or destroyed. The atom is the smallest unit of matter that can take part in a chemical reaction.
Q. Why is there a need to attain the law of conservation of mass?
According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants. The law of conservation of mass is useful for a number of calculations and can be used to solve for unknown masses, such the amount of gas consumed or produced during a reaction.
Q. What are the chemical reaction according to the law of conservation of mass?
Law of Conservation of Mass states that matter can neither be created nor be destroyed in any chemical reactions. In other words, Law of conservation of Mass states that in any chemical reactions the Mass of Products is always equal to the mass of Reactant.
Q. Why is it hard to prove the law of conservation of mass when a gas is produced?
It is difficult to prove the law of conservation of mass when a gas is produced because the gas molecules move quickly into the outside space and away…
Q. What are the limitations of the law of conservation of mass?
The other limitation of conservation of mass is according to Einstein’s theory, the relation between two quantities is given by E = mc2, which means that energy and mass are interconvertible. Therefore, for the law of conservation of mass to be valid mass and energy of the system must be conserved.
Q. Is the law of conservation of mass always true?
Mass is not conserved in chemical reactions. Mass is therefore never conserved because a little of it turns into energy (or a little energy turns into mass) in every reaction. But mass+energy is always conserved. Energy cannot be created out of nothing.
Q. Does the law of conservation of mass apply to every reaction?
Matter cannot be created or destroyed in chemical reactions. This is the law of conservation of mass. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Balanced chemical equations show that mass is conserved in chemical reactions.
Q. Can mass be destroyed?
The law implies that mass can neither be created nor destroyed, although it may be rearranged in space, or the entities associated with it may be changed in form. For example, in chemical reactions, the mass of the chemical components before the reaction is equal to the mass of the components after the reaction.