79 electrons
Q. How do you find core electrons?
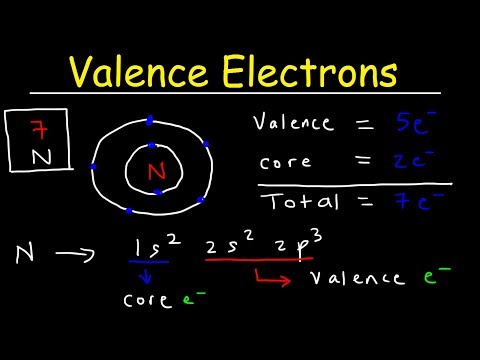
You can also find the core and valence electrons by determining or looking up the electron configurations of the main group elements. The atomic number is the number of protons in the nuclei of the atoms of an element. A neutral atom has the same number of electrons as protons. We can look at period 2 as an example.
Table of Contents
- Q. How do you find core electrons?
- Q. What is the purpose of core electrons?
- Q. What are the 2s electrons in nitrogen?
- Q. Is nh3 a sp2?
- Q. What is the complete electron configuration for nitrogen?
- Q. How many orbitals are in nitrogen?
- Q. How many vacant orbitals are present in nitrogen?
- Q. How many protons neutrons and electrons are in nitrogen 15?
- Q. How many electrons are in the anion of nitrogen?
- Q. Why is nitrogen an anion?
- Q. What happens when nitrogen gains 3 electrons?
- Q. Can nitrogen gain 3 electrons?
- Q. Why does nitrogen have a 3 charge?
Q. What is the purpose of core electrons?
The nucleus and the core electrons of an atom form the atomic core. Core electrons are tightly bound to the nucleus. Therefore, unlike valence electrons, core electrons play a secondary role in chemical bonding and reactions by screening the positive charge of the atomic nucleus from the valence electrons.
Q. What are the 2s electrons in nitrogen?
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.
Q. Is nh3 a sp2?
No, it only has 3 bonds and 1 lone pair. So it’s sp2.
Q. What is the complete electron configuration for nitrogen?
[He] 2s2 2p3
Q. How many orbitals are in nitrogen?
Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz).
Q. How many vacant orbitals are present in nitrogen?
Notice that for n = 1, there is a single s orbital, while for n = 2 there is one s orbital and three p orbitals.
Q. How many protons neutrons and electrons are in nitrogen 15?
The atomic number of the isotope nitrogen-15 is 7. How many protons, neutrons, and electrons make up an atom of nitrogen-15? Each atom of nitrogen-15 contains 7 protons, 8 neutrons, and 7 electrons.
Q. How many electrons are in the anion of nitrogen?
3 electrons
Q. Why is nitrogen an anion?
Nitrogen is neither a cation nor an anion because it is an atom and atoms are electrically neutral. Nitrogen has five valence electrons and it needs…
Q. What happens when nitrogen gains 3 electrons?
If Nitrogen gains three electrons the 2p orbitals will have 6 electrons giving 2p6 This creates the electron configuration of Neon making the atom much more stable than the initial or ground state.
Q. Can nitrogen gain 3 electrons?
Nitrogen, on the other hand, is in Group V and has five valence electrons, so it needs to gain three electrons to get a full valence shell. Thus the most stable state for nitrogen ions is the N3− ion.
Q. Why does nitrogen have a 3 charge?
nitrogen should have formal charge of three because the two s orbital electrons if participate in reaction, it would go totally unstable. The valence electrons of nitrogen in its compounds are all sp³ hybridized orbitals. The formal charge on N is usually -1 for an anion, 0 for a neutral compound, and +1 in cations.