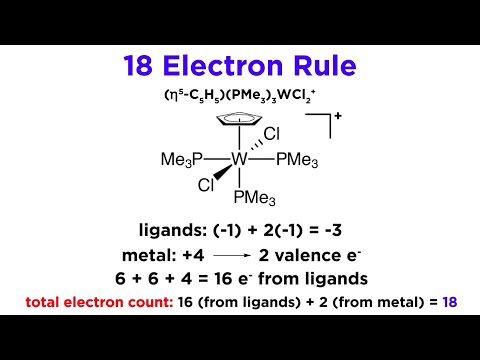
Q. How many electrons will be occupied in a cell out of 18 electrons?
Each orbital holds 2 electrons and you will indeed find that you can fir 18 electrons in. However, the orbitals must fill in a specific order.
Q. Can hold up to 18 electrons?
Most of the elements important in biology need eight electrons in their outermost shell in order to be stable, and this rule of thumb is known as the octet rule. Some atoms can be stable with an octet even though their valence shell is the 3n shell, which can hold up to 18 electrons.
Table of Contents
- Q. How many electrons will be occupied in a cell out of 18 electrons?
- Q. Can hold up to 18 electrons?
- Q. Why don’t we put 18 electrons in a third shell as it has the capacity of 18 electrons?
- Q. How many more electrons does oxygen need to fulfill the octet rule?
- Q. Is losing a hydrogen oxidation or reduction?
- Q. Is oxidation gaining or losing hydrogen?
- Q. Can you lose protons?
- Q. Can an atom lose a neutron?
- Q. What happens if you remove a proton?
- Q. What is it called when you gain or lose protons?
- Q. Why can protons never change?
- Q. Are hydrogen atoms positive or negative?
- Q. Is oxygen atom stable or unstable?
Q. Why don’t we put 18 electrons in a third shell as it has the capacity of 18 electrons?
In this sense the third shell can hold 8 electrons. In this sense the third shell can hold a total of 18 electrons. So the third shell can be considered to hold 8 or 18 electrons but in total the third shell can hold 18 electrons.
Q. How many more electrons does oxygen need to fulfill the octet rule?
Chemistry flashcards 51-100(Midterm Review)
| A | B |
|---|---|
| an octet = | 8 |
| the electron configuation of oxygen is 1s2 2s2 2p4. how many more electrons does oxygen need to satisfy the octet rule? | 2 |
| multiple covalent bonds may occur in atoms that contain carbon, nitrogen or | oxygen |
Q. Is losing a hydrogen oxidation or reduction?
Oxidation is loss of hydrogen. Reduction is gain of hydrogen.
Q. Is oxidation gaining or losing hydrogen?
Reduction
Q. Can you lose protons?
The only two ways by which atoms lose protons is through radioactive decay and nuclear fission. Both processes will only occur in atoms that have unstable nuclei. It is well known that radioactively occurs naturally and spontaneously.
Q. Can an atom lose a neutron?
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element.
Q. What happens if you remove a proton?
Adding or removing protons from a nucleus are types of nuclear reactions. As such, no series of chemical reactions can ever create gold. Chemical reactions change the number and shape of the electrons in an atom but leave the nucleus of the atom unchanged.
Q. What is it called when you gain or lose protons?
Sometimes atoms gain or lose electrons. The atom then loses or gains a “negative” charge. These atoms are then called ions. Positive Ion – Occurs when an atom loses an electron (negative charge) it has more protons than electrons.
Q. Why can protons never change?
It’s very important to keep in mind that the number of protons never changes when dealing with the ion of a chemical element. The only thing that changes is the number of electrons that surround the nucleus of the atom. The number of protons inside its nucleus will always remain constant.
Q. Are hydrogen atoms positive or negative?
Hydrogen is a positive ion. Hydrogen atoms consist of a proton in the nucleus surrounded by one electron.
Q. Is oxygen atom stable or unstable?
One oxygen atom is unstable since it has only 6 electrons in the outermost shell. For an atom to be stable it needs 8 electrons.