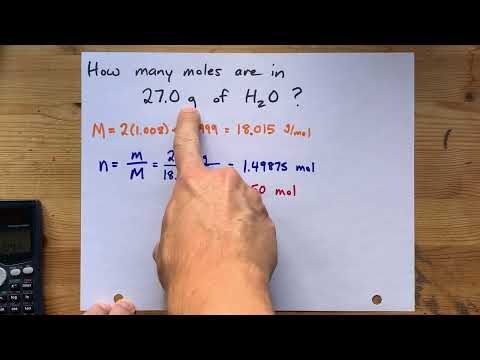Q. How many grams are in one mole of potassium sulfate?
174.2592 grams
Q. How many grams are in 1.15 moles of K2SO4?
You can view more details on each measurement unit: molecular weight of K2SO4 or grams This compound is also known as Potassium Sulfate. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles K2SO4, or 174.2592 grams.
Table of Contents
- Q. How many grams are in one mole of potassium sulfate?
- Q. How many grams are in 1.15 moles of K2SO4?
- Q. How many grams of fertilizer or potassium sulfate are there in 42.3 moles of potassium sulfate?
- Q. How many grams of potassium are in sulfite?
- Q. How many grams are in 3.3 moles of potassium sulfide K2S?
- Q. How many moles are in 100 grams of potassium sulfide?
- Q. How many moles are in 12 grams of potassium?
- Q. What is the total mass of 2.0 moles of hydrogen gas?
- Q. What is the mass of 2.5 moles of co2?
- Q. How much does 1 mole of hydrogen gas weigh?
- Q. What is the mass of 5 moles of hydrogen gas?
- Q. What is mass of 4 moles of hydrogen gas?
- Q. How do you calculate moles of hydrogen gas?
- Q. How many moles are present in 2.3 grams of sodium?
- Q. How many moles are 2.3 grams of phosphorus?
- Q. How many atoms are there in 0.1 moles of carbon?
- Q. How many moles are in water?
- Q. What is the mole of H2O?
- Q. How many moles are in 25 grams of NaOH?
- Q. What is the mass of 0.5 mole of CO2?
Q. How many grams of fertilizer or potassium sulfate are there in 42.3 moles of potassium sulfate?
The mass of 42.3 moles of potassium sulfate, m = 7,371.1557 grams.
Q. How many grams of potassium are in sulfite?
Potassium sulfite
| Names | |
|---|---|
| show SMILES | |
| Properties | |
| Chemical formula | K2SO3 |
| Molar mass | 158.26 g/mol |
Q. How many grams are in 3.3 moles of potassium sulfide K2S?
363.99 g
Q. How many moles are in 100 grams of potassium sulfide?
How many grams Potassium Sulfide in 1 mol? The answer is 110.2616. We assume you are converting between grams Potassium Sulfide and mole.
Q. How many moles are in 12 grams of potassium?
39 g/mol
Q. What is the total mass of 2.0 moles of hydrogen gas?
4.0 grams
Q. What is the mass of 2.5 moles of co2?
what is mass of 2.5 mole carbon dioxide ( given atomic mass of carbon=12 and oxygen =16 )A. 100B.
Q. How much does 1 mole of hydrogen gas weigh?
2 grams
Q. What is the mass of 5 moles of hydrogen gas?
Answer: Hydrogen’s molar mass is 1.008 g/mol. Hydrogen gas’s formula is H2 meaning its composed of two hydrogens therefore its molar mass is 2.016 g/mol. 5 mol H2 x 2.016g/mol = 10.08 g.
Q. What is mass of 4 moles of hydrogen gas?
8 g
Q. How do you calculate moles of hydrogen gas?
Use the mass of the hydrogen gas to calculate the gas moles directly; divide the hydrogen weight by its molar mass of 2 g/mole. For example, 250 grams (g) of the hydrogen gas corresponds to 250 g / 2 g/mole = 125 moles.
Q. How many moles are present in 2.3 grams of sodium?
2 mole.
Q. How many moles are 2.3 grams of phosphorus?
0.074 moles
Q. How many atoms are there in 0.1 moles of carbon?
Number of mol of carbon = 0.1 mol. Hence, the number of atoms in 0.1 mol of carbon atom is 0.1 NA atoms or 6.022×1022 atoms.
Q. How many moles are in water?
The number of atoms is an exact number, the number of mole is an exact number; they do not affect the number of significant figures. The average mass of one mole of H2O is 18.02 grams. This is stated: the molar mass of water is 18.02 g/mol.
Q. What is the mole of H2O?
Molar mass H2O = (2×atomic mass H) + (1×atomic mass O) = (2×1.00794) + (1×15.9994) = 18.02 • 1 H2O molecule has a mass of 18.02 amu. 1 mole of H2O molecules has a mass of 18.02 grams.
Q. How many moles are in 25 grams of NaOH?
The answer is 39.99711. We assume you are converting between grams NaOH and mole. You can view more details on each measurement unit: molecular weight of NaOH or mol This compound is also known as Sodium Hydroxide. The SI base unit for amount of substance is the mole.
Q. What is the mass of 0.5 mole of CO2?
0.5 mole CO2=0. 5×44=22 gm.