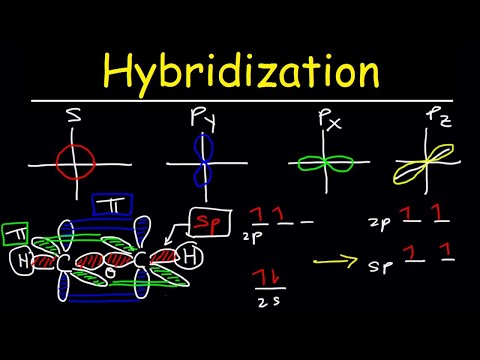
To accommodate these two electron domains, two of the Be atom’s four valence orbitals will mix to yield two hybrid orbitals. When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals. The Be atom had two valence electrons, so each of the sp orbitals gets one of these electrons.
Q. How many hybrid orbitals are there?
The carbon is bonded to two other atoms, that means it needs two hybrid orbitals, aka sp. An easy way to figure out what hybridization an atom has is to just count the number of atoms bonded to it and the number of lone pairs. Double and triple bonds still count as being only bonded to one atom.
Table of Contents
- Q. How many hybrid orbitals are there?
- Q. How do you know how many hybrid orbitals are formed?
- Q. How many sp2 hybrid orbitals are formed?
- Q. Is oxygen a sp2 or sp3?
- Q. What is the formula to calculate hybridization?
- Q. Is sp3 more stable than sp2?
- Q. Are double bonds sp2?
- Q. Is triple bond a sp3?
- Q. Does octane have a triple bond?
- Q. Can sp3 form pi bonds?
- Q. Is sp3 a sigma or pi?
- Q. How many pi bonds can sp3 make?
- Q. How many pi bonds are there in sp3?
- Q. Is a triple bond sigma or pi?
- Q. Is Sigma bond stronger than pi?
- Q. What is a sigma and pi bond?
- Q. What is sigma bond example?
- Q. How can you tell the difference between sigma and pi bonds?
Q. How do you know how many hybrid orbitals are formed?
When atoms share electrons with other atoms to form chemical bonds, the orbitals that contain the electrons involved in the bonding merge to form a “hybrid” orbital. Count the number of electron domains of the central atom in the molecule by noting the number of unpaired electrons and bonds on the central atom.
Q. How many sp2 hybrid orbitals are formed?
three
Q. Is oxygen a sp2 or sp3?
The oxygen is sp3 hybridized which means that it has four sp3 hybrid orbitals. One of the sp3 hybridized orbitals overlap with s orbitals from a hydrogen to form the O-H signma bonds. One of the sp3 hybridized orbitals overlap with an sp3 hybridized orbital from carbon to form the C-O sigma bond.
Q. What is the formula to calculate hybridization?
Hybridization=1/2[V+M-C+A] Let us put the values according to the formula. The hybridization number is equal to 7. Now we can say that hybridization is sp3d3. Alternatively, we can also determine the hybridization of I3- by knowing the number of bond pairs and lone pairs.
Q. Is sp3 more stable than sp2?
The electrons of an sp3 hybridized atom are known to be farther from the nucleus than those in sp2 hybridized species. Therefore, sp2 hybrid species are more stable than sp3 hybrid species. This is because the stability is greater when the electrons are close to the nucleus.
Q. Are double bonds sp2?
If you have one double bond, it is sp2. If you have two double bonds it is sp. So each double bond bring the degree of the p level down by 1.
Q. Is triple bond a sp3?
Since each carbon atom is sp hybridized, then each carbon atom has two unhybridized p atomic orbitals. The two C−H sigma bonds are formed from overlap of carbon sp hybrid orbitals with hydrogen 1s atomic orbitals. The triple bond is composed of one σ bond and two π bonds. The carbon atoms are sp3 hybridized.
Q. Does octane have a triple bond?
Because the triple bond is located on the end of the carbon chain, these are sometimes referred to, as “terminal alkynes.” The parent name of this compound is octane and the triple bond begins on C3. So, the parent name of the compound is: 3-octyne.
Q. Can sp3 form pi bonds?
If a carbon atom is sp3 hybridized it can only form sigma bonds. With sp2 hybridization you can form a single pi bond with the unhybridized 2p orbitals and the sigma bond would be between sp2 orbitals, forming a double bond.
Q. Is sp3 a sigma or pi?
The number of orbitals taking part in hybridization is the number of sigma bonds made around the central atom. In sp3, sp3d and sp3d2 no pi bond is present as it contains only a single covalent bond.
Q. How many pi bonds can sp3 make?
Pi bonds can only be formed between two p orbitals. As a result of all this, an sp hybridised carbon for example can form 2 sigma bonds (because it has to hybridised orbitals, the s and the p) and 2 pi bonds (because it has to p orbitals). An sp3 hybridised carbon can form 4 sigma bonds and 0 pi bonds and so on.
Q. How many pi bonds are there in sp3?
Therefore, sp3 hybridization in carbon will feature 0 pi bonds.
Q. Is a triple bond sigma or pi?
In general, single bonds between atoms are always sigma bonds. Double bonds are comprised of one sigma and one pi bond. Triple bonds are comprised of one sigma bond and two pi bonds.
Q. Is Sigma bond stronger than pi?
Sigma bonds are significantly stronger than pi bonds. This is because sigma bonds allow for electron density to be concentrated to a much larger degree between the two nuclei. You can also conceptualize that pi bonds are weaker simply because we know those electrons are in a higher-energy state.
Q. What is a sigma and pi bond?
Sigma and pi bonds are chemical covalent bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma bonds are formed by end-to-end overlapping and Pi bonds are when the lobe of one atomic orbital overlaps another. Generally sigma bonds are stronger than pi bonds.
Q. What is sigma bond example?
The bond between two hydrogen atoms is an example of sigma bonding. The bonds between the sp3 orbitals of hybridized carbon and the s orbitals of hydrogen in methane are also example of sigma bonds.
Q. How can you tell the difference between sigma and pi bonds?
| Difference Between Sigma and Pi bond | |
|---|---|
| During the bonding between two given atoms, Only one sigma bond is formed. | Here two pi bonds can exist between two atoms. |
| Sigma bonds are known to have cylindrical charge symmetry around the axis of the bond. | No symmetry exists in pi bonds. |