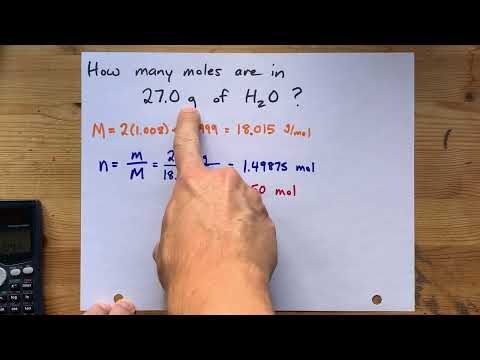Q. How many moles are in CaF2?
You can view more details on each measurement unit: molecular weight of CaF2 or grams This compound is also known as Calcium Fluoride. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles CaF2, or 78.0748064 grams.
Q. How many moles are in 17.5 g of CaF2?
The answer is 78.0748064. We assume you are converting between grams CaF2 and mole. You can view more details on each measurement unit: molecular weight of CaF2 or mol This compound is also known as Calcium Fluoride. The SI base unit for amount of substance is the mole.
Table of Contents
- Q. How many moles are in CaF2?
- Q. How many moles are in 17.5 g of CaF2?
- Q. How many moles of CaF2 are in 75.0 grams?
- Q. How many moles are in 39 g of CaF2?
- Q. How many grams are there in 3 mol NaNO3?
- Q. How do you find the number of moles?
- Q. How do you find how many grams are in a mole?
- Q. How do you find the number of moles from volume?
- Q. How do you find the number of moles at STP?
- Q. What would the volume of this oxygen be at STP?
- Q. What is the mass of a mole?
- Q. What is Mole concept example?
- Q. How do you convert moles to mass?
- Q. How many grams are in 1.50 moles of KMnO4?
- Q. How many moles are in 25 grams of KMnO4?
- Q. How many grams are in 1.70 mol of KMnO4?
- Q. What is the mass of 0.50 moles of H2SO4?
- Q. What is the chemical formula of sulfuric acid?
- Q. How many moles are in 100 grams of potassium?
- Q. How many grams are in a mole of silver nitrate?
- Q. How many grams are in 3.8 moles of AgNO3?
- Q. What is the weight of 0.02 moles of AgNO3?
- Q. What is the total mass of 2.0 moles of hydrogen gas?
- Q. How do you find moles of AgNO3?
- Q. How many moles are in 1g of AgNO3?
- Q. How many grams is cuno32?
- Q. What is the molar mass of CaF2?
- Q. What is the mass of 2 moles of oxygen?
- Q. What is the mass of 1 mole of?
- Q. What is the mass of 5 moles of oxygen gas?
- Q. How many grams are in four moles of oxygen?
- Q. How many grams are in a mole of oxygen gas?
- Q. How many moles are in 54 grams of aluminum?
- Q. How many grams are in 1 mole of aluminum?
- Q. How many moles are in 10g of aluminum?
- Q. How many moles are in 95.0 grams of aluminum?
- Q. How do I convert moles to grams?
- Q. How many moles of aluminum are needed?
- Q. How many moles of glucose does C6H12O6?
- Q. How many moles of glucose can be burned biologically when 10 moles of oxygen is available?
Q. How many moles of CaF2 are in 75.0 grams?
0.960 moles
Q. How many moles are in 39 g of CaF2?
So CaF2 ould have . 5 as the amount of moles.
Q. How many grams are there in 3 mol NaNO3?
We assume you are converting between moles NaNO3 and gram. You can view more details on each measurement unit: molecular weight of NaNO3 or grams This compound is also known as Sodium Nitrate. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles NaNO3, or 84.99467 grams.
Q. How do you find the number of moles?
So in order to calculate the number of moles of any substance present in the sample, we simply divide the given weight of the substance by its molar mass. Where ‘n’ is the number of moles, ‘m’ is the given mass and ‘M’ is the molar mass.
Q. How do you find how many grams are in a mole?
Divide the mass of the substance in grams by its molecular weight. This will give you the number of moles of that substance that are in the specified mass. For 12 g of water, (25 g)/(18.015 g/mol) = 0.666 moles.
Q. How do you find the number of moles from volume?
Multiply the volume by the density to get the mass. Divide the mass by the molar mass to get the number of moles.
Q. How do you find the number of moles at STP?
To go from grams to moles, divide the grams by the molar mass. 600 g58.443 g/mol = 10.27 mol of NaCl. It has been found that 1 mol of any gas at STP (Standard Temperature and Pressure = 0 °C and 1 atm) occupies 22.4 L. 6.00 mol×22.4 L1 mol = 134.4 L of CO.
Q. What would the volume of this oxygen be at STP?
Finally, use the fact that one mole of oxygen would occupy a volume of 22.4 L at STP to figure out the volume of the oxygen in this question at STP. The answer is 0.338 L (338 mL), but you will have to show the calculations to receive credit on your lab report.
Q. What is the mass of a mole?
The molar mass of an element (or compound) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) (see Figure 3.5)….The Mole.
| Element | C |
|---|---|
| Average Atomic Mass (amu) | 12.01 |
| Molar Mass (g/mol) | 12.01 |
| Atoms/Mole | 6.022 × 1023 |
Q. What is Mole concept example?
The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. A mole is defined as the amount of substance containing the same number of discrete entities (atoms, molecules, ions, etc.) as the number of atoms in a sample of pure 12C weighing exactly 12 g.
Q. How do you convert moles to mass?
Multiply the molecular weight by the number of moles for the substance. The molecular weight is the number of grams per mole for the substance and gives the conversion factor for moles to grams for that particular substance. So, one mole of water has a mass of 18.02 grams (1 mol H2O x 18.02 g/mol = 18.02 g).
Q. How many grams are in 1.50 moles of KMnO4?
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles KMnO4, or 158.033949 grams.
Q. How many moles are in 25 grams of KMnO4?
The answer is 158.033949. We assume you are converting between grams KMnO4 and mole. You can view more details on each measurement unit: molecular weight of KMnO4 or mol This compound is also known as Potassium Permanganate.
Q. How many grams are in 1.70 mol of KMnO4?
269g
Q. What is the mass of 0.50 moles of H2SO4?
The mass of 0.5 moles of is 49 u.
Q. What is the chemical formula of sulfuric acid?
H₂SO₄
Q. How many moles are in 100 grams of potassium?
How many grams Potassium in 1 mol? The answer is 39.0983.
Q. How many grams are in a mole of silver nitrate?
169.87 grams
Q. How many grams are in 3.8 moles of AgNO3?
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles AgNO3, or 169.8731 grams.
Q. What is the weight of 0.02 moles of AgNO3?
Chemical Identifiers
| CAS | 7761-88-8 |
|---|---|
| Molecular Formula | AgNO3 |
| Molecular Weight (g/mol) | 169.872 |
| MDL Number | 3414 |
| InChI Key | SQGYOTSLMSWVJD-UHFFFAOYSA-N |
Q. What is the total mass of 2.0 moles of hydrogen gas?
4.0 grams
Q. How do you find moles of AgNO3?
But in solution, you find moles of AgNO3. That is 0.0132 g AgNO3 x 1 mol AgNO3/169.9 g = 7.77×10-5 moles. Since there is 1 mole Ag and 1 mole NO3 per mole of AgNO3, this is also the number of moles of Ag+ and NO3- ions.
Q. How many moles are in 1g of AgNO3?
0.0058867472248402 mole
Q. How many grams is cuno32?
187.5558 grams
1 moles
Q. What is the molar mass of CaF2?
78.07 g/mol
The unit is denoted by mol.
- The formula for the number of moles formula is expressed as.
- Given.
- Number of moles formula is.
- Number of moles = Mass of substance / Mass of one mole.
- Number of moles = 95 / 86.94.
Q. What is the mass of 2 moles of oxygen?
63.9976 g
Q. What is the mass of 1 mole of?
And whereas one sodium atom has an approximate mass of 23 u, 1 mol of Na atoms has an approximate mass of 23 grams. One mole of a substance has the same mass in grams that one atom or molecule has in atomic mass units….
| 1 Ba molar mass: | 1 × 137.3 g = | 137.3 g |
|---|---|---|
| 2 H molar mass: | 2 × 1.01 g = | 2.02 g |
| Total: | 171.32 g |
Q. What is the mass of 5 moles of oxygen gas?
160 g
Q. How many grams are in four moles of oxygen?
128 g
Q. How many grams are in a mole of oxygen gas?
16 g
Q. How many moles are in 54 grams of aluminum?
2 moles
Q. How many grams are in 1 mole of aluminum?
26.982 g
Q. How many moles are in 10g of aluminum?
The answer is 26.981538. We assume you are converting between grams Aluminum and mole. You can view more details on each measurement unit: molecular weight of Aluminum or mol The molecular formula for Aluminum is Al. The SI base unit for amount of substance is the mole.
Q. How many moles are in 95.0 grams of aluminum?
mol Al. 2.10 mol FeCl2 = molecules FeCl2.
Q. How do I convert moles to grams?
You have three steps to convert mole values to grams.
- Calculate how many moles are mentioned in the question.
- Find the molar mass of the substance.
- Multiply both the values.
Q. How many moles of aluminum are needed?
1 Expert Answer From the balanced equation, it can be seen that 4 moles Al are needed to produce 2 moles Al2O3, thus, 3 moles Al2O3 x 4 moles Al/2 moles Al2O3 = 6 moles Al needed.
Q. How many moles of glucose does C6H12O6?
1 molecule of glucose contains 6 atoms of C, 12 atoms of H, and 6 atoms of O • 1 mole of glucose contains 6 moles of C atoms, 12 moles of H atoms, and 6 moles of O atoms.
Q. How many moles of glucose can be burned biologically when 10 moles of oxygen is available?
26 Cards in this Set
| In any chemical reaction, the quantities that are preserved are___? | mass and number of atoms or (always equal?) |
|---|---|
| How many moles of glucose, C H O , can be “burned” biologically when 10.0 mol of oxygen is available?C6H12O6 (s)+6O2 (g)–> 6CO2 (g)+6H2 O(l) | 1.67 mol |