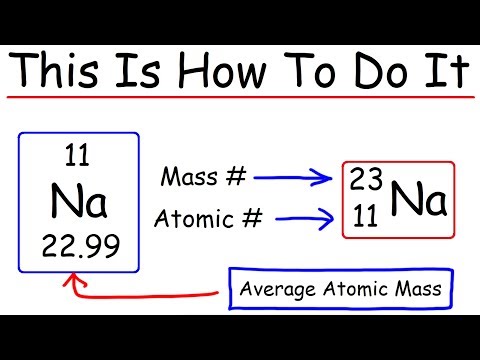
Q. How many neutrons are there in oxygen?
Oxygen-16 (16O) is a stable isotope of oxygen, having 8 neutrons and 8 protons in its nucleus. It has a mass of 15.99491461956 u….Oxygen-16.
| General | |
|---|---|
| Protons | 8 |
| Neutrons | 8 |
| Nuclide data | |
| Natural abundance | 99.76% |
Q. How many protons neutrons and electrons does a neutrally charged oxygen atom have?
An oxygen atom has 8 positive protons and 8 neutrons in its nucleus, and 8 negative electrons speeding around the nucleus.
Table of Contents
- Q. How many neutrons are there in oxygen?
- Q. How many protons neutrons and electrons does a neutrally charged oxygen atom have?
- Q. Can oxygen have 9 electrons?
- Q. What does 1amu mean?
- Q. What is the value of 1 am?
- Q. Are Amu and G the same?
- Q. Is 1u equal to 1g?
- Q. How many amu are in a mol?
- Q. Is Dalton and Amu the same?
- Q. How do you convert AMU to KG?
- Q. Who invented the mole?
- Q. Who gave Avogadro number?
- Q. How is mole eaten?
- Q. Why is it called Avogadro’s number?
- Q. Why is it called a mole?
- Q. What is the mass of one mole of oxygen?
- Q. How many grams is a mol?
- Q. How much is in a mol?
Q. Can oxygen have 9 electrons?
Answer 9: Oxygen is atomic number 8 on the periodic table, which means it has 8 protons! This means that a neutral oxygen atom will have 8 electrons (but this number can change!
Q. What does 1amu mean?
atomic mass unit
Q. What is the value of 1 am?
Explanation: 1 atomic mass unit (a.m.u.) is the mass of a proton or a neutron which is equal to 1.6726219×10−27kg .
Q. Are Amu and G the same?
The main difference between amu and grams is that amu is used to express the mass in atomic level whereas gram is used as a metric unit of mass.
Q. Is 1u equal to 1g?
1 u is mass of 1 hydrogen atom. Also 6.02 × 10^23 atoms of hydrogen have 1 gram mass. So solve by basic unitary method to find what mass would 1 atom would have, which is 1/N (N is avogadro’s constant).
Q. How many amu are in a mol?
Chemical Computations with Avogadro’s Number and the Mole Another property of Avogadro’s number is that the mass of one mole of a substance is equal to that substance’s molecular weight. For example, the mean molecular weight of water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams.
Q. Is Dalton and Amu the same?
The dalton is simply a renaming of the unified atomic mass unit, so they have equal values—there is no distinction between the two units other than name and symbol, so the two names are synonymous as are the two unit symbols.
Q. How do you convert AMU to KG?
The mass of an element or molecule in atomic mass units is equal to the mass of a mole of the same particles in grams. Divide the number by 1,000 to get the molar mass in kilograms.
Q. Who invented the mole?
Amedeo Avogadro
Q. Who gave Avogadro number?
physicist Jean Baptiste Perrin
Q. How is mole eaten?
Mole is commonly served with chicken in a dish called pollo en mole. It is also served with enmoladas which are enchiladas prepared with mole instead of salsa. It may sound strange, but you should try scrambled eggs with mole.
Q. Why is it called Avogadro’s number?
It is named after the Italian scientist Amedeo Avogadro. Thus, the Avogadro constant NA is the proportionality factor that relates the molar mass of a substance to the average mass of one molecule, and the Avogadro number is also the approximate number of nucleons in one gram of ordinary matter.
Q. Why is it called a mole?
The mole is a unit used in chemistry that is equal to Avogadro’s number. It is the number of carbon atoms in 12 grams of the isotope carbon-12. The word mole comes from the word molecule.
Q. What is the mass of one mole of oxygen?
15.998 grams
Q. How many grams is a mol?
114.818 grams
Q. How much is in a mol?
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro’s number or Avogadro’s constant.