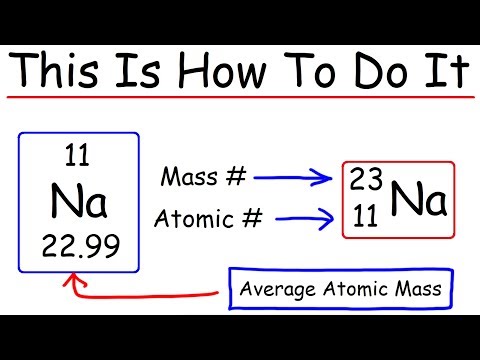
Q. How many nucleus does sodium have?
| Name | Sodium |
|---|---|
| Number of Protons | 11 |
| Number of Neutrons | 12 |
| Number of Electrons | 11 |
| Melting Point | 97.88° C |
Q. How many protons electrons and neutrons does sodium have?
So sodium has 11 protons and 11 electrons. The mass number is 23. The number of neutrons = mass number – atomic number. The number of neutrons = 23 – 11 = 12.
Q. How many neutrons are in a sodium atom?
11
Table of Contents
- Q. How many nucleus does sodium have?
- Q. How many protons electrons and neutrons does sodium have?
- Q. How many neutrons are in a sodium atom?
- Q. What is the nucleus of sodium?
- Q. What type of structure is sodium?
- Q. What is the charge on a sodium nucleus?
- Q. Why does an atom of sodium have no overall charge?
- Q. Which subatomic particle is the lightest?
- Q. What is magnesium’s charge?
- Q. Why does CU have a 2 charge?
- Q. What is the charge of H?
- Q. Which elements have a charge?
- Q. How do I know the charge of an element?
- Q. What is the charge of Group 4 elements?
- Q. Is carbon plus or minus 4?
- Q. Does Group 4 gain or lose electrons?
- Q. How many electrons does group 4 have?
- Q. Is Group 4 metal or nonmetal?
- Q. What kind of elements has the greatest tendency to attract?
- Q. What is Group 5 called?
- Q. What is Group 4 called?
- Q. What is Group 2 called?
- Q. Which is the lightest element in the world?
- Q. What is the heaviest element in the universe?
- Q. Which is the smallest element?
- Q. What is Earth’s most abundant element?
- Q. What is the rarest element in the universe?
- Q. Which metal is found most in earth?
- Q. What are the 5 most common elements on earth?
Q. What is the nucleus of sodium?
Almost all sodium on Earth is sodium-23, where the number refers to the 11 protons and 12 neutrons that make up its nucleus. Yet those 23 particles do not encompass all that can or could be sodium. Technically, any nucleus with 11 protons is sodium.
Q. What type of structure is sodium?
ionic structure
Q. What is the charge on a sodium nucleus?
+1
Q. Why does an atom of sodium have no overall charge?
An atom contains equal numbers of protons and electrons . Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Every sodium atom has 11 protons and 11 electrons. It has 11 positive charges and 11 negative charges.
Q. Which subatomic particle is the lightest?
Electron
Q. What is magnesium’s charge?
A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is Mg2+, and it is called a magnesium ion.
Q. Why does CU have a 2 charge?
Since the energies of the 4s and the 3d electrons in copper is nearly identical, it is also possible to remove both 4s electrons( instead of moving it to a d orbital. This makes the cupric or Cu(II) 2+ ion.
Q. What is the charge of H?
Q. Which elements have a charge?
Carbon is a solid and oxygen is a gas; when they combine they become carbon dioxide a gas. Generally, metals on the Periodic Table of the Elements have a positive charge (a positive ion) and the nonmetals have a negative charge (a negative ion). There are several exceptions to this rule.
Q. How do I know the charge of an element?
To find the ionic charge of an element you’ll need to consult your Periodic Table. On the Periodic Table metals (found on the left of the table) will be positive. Non-metals (found on the right) will be negative.
Q. What is the charge of Group 4 elements?
Defining group 4 as the second group in the transition metals: Most transition metals usually have a 2+ ion (with some exceptions not in this group). They may also have different charges, since their missing electrons are in the d-orbital, the atoms have more options as to what will happen.
Q. Is carbon plus or minus 4?
General properties of the group
| carbon | |
|---|---|
| oxidation states | −4, (+2), +4 |
| mass number of most common isotopes (terrestrial abundance, percent) | 12 (98.89), 13 (1.11) |
| radioactive isotopes (mass numbers) | 8–11, 14–22 |
| heat of fusion (calories per mole/kilojoules per mole) | 25,100 (105) |
Q. Does Group 4 gain or lose electrons?
Group 4 elements have 4 valence electrons. The non-metals in this family react by gaining 4 extra electrons through the formation of covalent bonds (sharing bonds).
Q. How many electrons does group 4 have?
Four covalent bonds. Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent….The number of valence electrons.
| Periodic table block | Periodic table group | Valence electrons |
|---|---|---|
| p | Group 14 (IV) (carbon group) | 4 |
Q. Is Group 4 metal or nonmetal?
Like silicon, germanium is used as a semiconductor, and is widely used in the computer industry. Silicon and germanium are both metalloids, having some characteristics of both metals and nonmetals….Group 4A.
| 3A | (13) |
|---|---|
| 4A | (14) |
| 5A | (15) |
| 6A | (16) |
Q. What kind of elements has the greatest tendency to attract?
The correct answer of this question is fluorine. Explanation: The tendency of attracting electrons is known as the electronegativity.
Q. What is Group 5 called?
Pnictogens
Q. What is Group 4 called?
Group 4 is the second group of transition metals in the periodic table. It contains the four elements titanium (Ti), zirconium (Zr), hafnium (Hf), and rutherfordium (Rf). The group is also called the titanium group or titanium family after its lightest member. All the group 4 elements are hard, refractory metals.
Q. What is Group 2 called?
Group 2A (or IIA) of the periodic table are the alkaline earth metals: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). In most cases, the alkaline earth metals are ionized to form a 2+ charge.
Q. Which is the lightest element in the world?
Hydrogen
Q. What is the heaviest element in the universe?
uranium
Q. Which is the smallest element?
helium
Q. What is Earth’s most abundant element?
Iron
Q. What is the rarest element in the universe?
Astatine is the rarest naturally occurring element.
Q. Which metal is found most in earth?
Aluminum
Q. What are the 5 most common elements on earth?
The mass-abundance of the nine most abundant elements in the Earth’s crust is approximately: oxygen 46%, silicon 28%, aluminum 8.3%, iron 5.6%, calcium 4.2%, sodium 2.5%, magnesium 2.4%, potassium 2.0%, and titanium 0.61%.