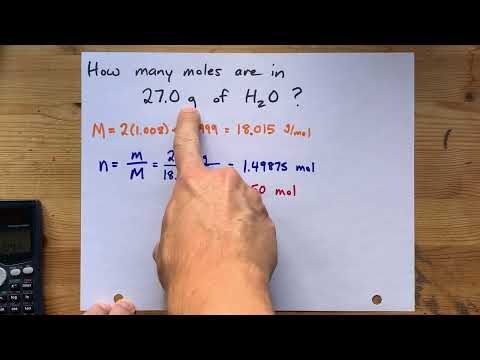Q. How many objects are in a mole?
1 mol = 6.022 x 1023 items, particles, things, etc.
Q. What is a mole in chemistry?
Mole, also spelled mol, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles. …
Table of Contents
- Q. How many objects are in a mole?
- Q. What is a mole in chemistry?
- Q. Is Mole a molecule?
- Q. How much is a mole of an object?
- Q. What is the formula for mole?
- Q. How tall is a mole of pennies?
- Q. How much money would you have if you had a mole of pennies?
- Q. How big is a mole of sand?
- Q. How much is a mole of Dimes?
- Q. How big is a mole of donuts?
- Q. How long would it take to spend a mole of dollars?
- Q. How much does a mole of hydrogen weigh?
- Q. How many grams of water are in a mole?
- Q. What size would a mole of basketballs be?
- Q. How big is a mole of water?
- Q. How many moles are in 100 g of water?
Q. Is Mole a molecule?
A mole of a substance is equal to as many molecules of that substance as there are atoms of carbon-12 in exactly 12 g of carbon-12. The word mole applies not just to molecules but also to atoms; in practice, we speak of a mole of helium atoms as well as of a mole of water molecules.
Q. How much is a mole of an object?
A mole is defined as the amount of matter that contains as many objects as the number of atoms in exactly 12 grams of 12C. Various experiments have determined that this number is… This is usually abbreviated to simply 6.02 x 1023, and is known as Avogadro’s number.
Q. What is the formula for mole?
Avogadro’s number is a very important relationship to remember: 1 mole = 6.022×1023 6.022 × 10 23 atoms, molecules, protons, etc. To convert from moles to atoms, multiply the molar amount by Avogadro’s number. To convert from atoms to moles, divide the atom amount by Avogadro’s number (or multiply by its reciprocal).
Q. How tall is a mole of pennies?
1 mole of pennies = 6.02×1023 pennies (that’s a lot of pennies). How many dollars is that? Now, how many years would this amount last if you spent it at a rate of $1 billion a second? So, 1 billion dollars is 1×109 dollars.
Q. How much money would you have if you had a mole of pennies?
If one mole of pennies were distributed equally among 6 billion people, each person would get 1×1014 pennies or 1×1012 dollars (1,000,000,000,000 = 1 trillion dollars)!
Q. How big is a mole of sand?
Now imagine a mole of sand particles. That would be 6.02 x 1023 grains of sand. How big would it be? If you put all of that sand in a cube shaped box, it would be about 4,400 meters tall, 4,400 meters wide and 4,400 meters deep.
Q. How much is a mole of Dimes?
For starters, you should know that in order to have 1 mole of dime coins, you need to have 6.022⋅1023 dime coins.
Q. How big is a mole of donuts?
So the mole is the title used for the amount 6.022 x 1023 much the same way the word “dozen” is used for the amount 12. So if you had a mole of donuts you would have 6.022 x 1023 donuts and a serious stomach ache.
Q. How long would it take to spend a mole of dollars?
How many years would it take you to spend 1 mole of dollars, if you spend at a rate of $1 billion per second ? = 19,089,294.77 years over 19 million years!!! What would you be called if you have 1 mole of dollars? a mole-ionaire!
Q. How much does a mole of hydrogen weigh?
One MOLE of hydrogen atoms contains the same number of atoms as the number of hydrogen molecules in one MOLE of hydrogen molecules, i.e., Avagadros number. However, one mole of hydrogen atoms has a mass of 1 gram while one MOLE of hydrogen molecules has a mass of 2 grams.
Q. How many grams of water are in a mole?
18.02 grams
Q. What size would a mole of basketballs be?
Activity: How big is a Mole? A mole is 6.02 x 1023 of anything. A mole of donuts is 6.02 x 1023 donuts, and a mole of basketballs is 6.02 x 1023 basketballs—and that’s a lot of basketballs! A mole of basketballs would just about fit into a ball bag the size of the Earth!
Q. How big is a mole of water?
Because the mole contains so many units, they’re most often used in chemistry is a way of measuring really really small things like atoms or molecules. So a mole of water is 6.02 x 1023 molecules of water, which works out to be about 18 grams, or 18 mL.
Q. How many moles are in 100 g of water?
5.55 moles