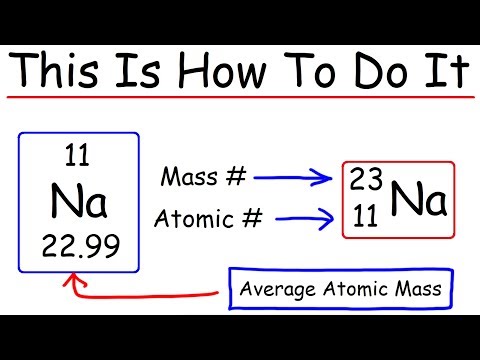
Q. How many protons neutrons and electrons does copper 66 have?
There are 29 protons, 35 neutrons, and 29 electrons in a copper atom.
Q. How many protons electrons and neutrons are in copper 63?
This means that the copper atom has 29 protons and 34 neutrons in its nucleus (29 + 34 = 63)….
Table of Contents
- Q. How many protons neutrons and electrons does copper 66 have?
- Q. How many protons electrons and neutrons are in copper 63?
- Q. What is the number of electrons of copper?
- Q. What is the number of protons in copper?
- Q. What would be the full electron configuration for copper?
- Q. Why is cu2+ more common than Cu+?
- Q. Which copper charge is more common?
- Q. What is meant by disproportionation of the oxidation state give example?
- Q. Why do transition metals act as catalysts?
- Q. Are Group 1 metals used as catalysts?
- Q. Why is Aluminium in period 3?
- Q. What is the atomic number of elements of Period 3?
| atom/element | copper (Cu) |
|---|---|
| mass number | 63 |
| number of protons | 29 |
| number of neutrons | 34 |
| number of electrons | 29 |
Q. What is the number of electrons of copper?
29 electrons
Q. What is the number of protons in copper?
29
Q. What would be the full electron configuration for copper?
[Ar] 3d¹⁰ 4s¹
Q. Why is cu2+ more common than Cu+?
Stability depends on the hydration energy (enthalpy) of the ions when they bond to the water molecules. The Cu2+ ion has a greater charge density than Cu+ ion and thus forms much stronger bonds releasing more energy.
Q. Which copper charge is more common?
Table of Common Element Charges
| Number | Element | Charge |
|---|---|---|
| 28 | nickel | 2+ |
| 29 | copper | 1+, 2+ |
| 30 | zinc | 2+ |
| 31 | gallium | 3+ |
Q. What is meant by disproportionation of the oxidation state give example?
When a particular oxidation state becomes less stable relative to other oxidation states, one lower, one higher, it is said to undergo disproportionation. For example, manganese (VI) becomes unstable relative to manganese(VII) and manganese (IV) in acidic solution.
Q. Why do transition metals act as catalysts?
Transition metals and their compounds are often good catalysts. Transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to adsorb other substances on to their surface and activate them in the process.
Q. Are Group 1 metals used as catalysts?
Alkali metals (especially K) are all highly reactive and electropositive, and their carbonates can be used directly as catalysts or supported on other materials such as alumina (Abu El-Rub et al., 2004).
Q. Why is Aluminium in period 3?
In the whole of period 3, the outer electrons are in 3-level orbitals. That increases ionisation energies still more as you go across the period. The fall at aluminium. You might expect the aluminium value to be more than the magnesium value because of the extra proton.
Q. What is the atomic number of elements of Period 3?
Atomic number of Chlorine is 17. Was this answer helpful?