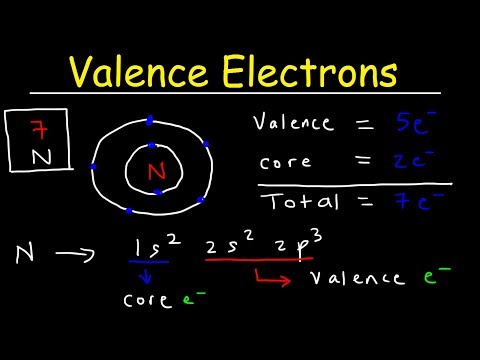
Q. How many valence electrons are in bicarbonate?
Step 1: The total number of valence electrons of the atoms is 23 plus one for the negative charge, giving a total of 24. Step 2: We can deduce the skeletal structure of the bicarbonate ion by realizing that C is less electronegative than O.
Q. How many valence electrons are in pcl4+?
In the Lewis structure for PCl4- there are a total of 34 valence electrons. Four pairs will be used in the chemical bonds between the P and F.
Table of Contents
- Q. How many valence electrons are in bicarbonate?
- Q. How many valence electrons are in pcl4+?
- Q. What is the shape of PCl4+?
- Q. What is the shape of PCl4 +?
- Q. What shape is pcl6?
- Q. What is the Vsepr shape of CBr4?
- Q. What is the bond angle of CBr4?
- Q. What is the bond angle of sih4?
- Q. Are all bond angles in CH2Cl2 identical?
- Q. How do you determine bond angles?
- Q. What are the bond angles?
- Q. What is meant by dihedral angle?
- Q. Which has the largest bond angle?
- Q. Which molecule has a 120 degree bond angle?
Q. What is the shape of PCl4+?
tetrahedral
Q. What is the shape of PCl4 +?
The shapes of PCl4+,PCl4− and AsCl5 are tetrahedral, see-saw and trigonal bipyramidal, respectively.
Q. What shape is pcl6?
Electron pair geometry different from the molecular geometry. chlorine(V) fluoride ClF5 (chlorine pentafluoride) is a square pyramid shaped molecule, with F-Cl-F bond angles of ~90o (just less than 90o?) and ~180o (actually 175o).
Q. What is the Vsepr shape of CBr4?
Q. What is the bond angle of CBr4?
around 109.5 degrees
Q. What is the bond angle of sih4?
109.5˚
Q. Are all bond angles in CH2Cl2 identical?
This is because CH4 has all the identical hydrogen atoms around carbon, whereas CH2Cl2 has 2 H and 2 Cl. This is reflected in the slight asymmetry in the molecular shape of the latter. This means that the bond angles and bond lengths in CH2Cl2 are not identical; however, all bond angles are identical in CH4.
Q. How do you determine bond angles?
1 Answer
- Write the Lewis dot structure for the molecule. Assume that you must determine the bond angles in BF3 .
- Use the steric number and VSEPR theory to determine the electron domain geometry of the molecule.
- Use the VSEPR shape to determine the angles between the electron domains.
Q. What are the bond angles?
A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei.
Q. What is meant by dihedral angle?
A dihedral angle is defined as the angle between two planes, both of which pass through the same bond. One of the planes also contains one of the additional bonds formed by one of the bond termini, and the other plane contains one of the additional bonds formed by the other terminus.
Q. Which has the largest bond angle?
Cl2O has largest bond angle because the more lone pair-bond pair repulsion increases the more the bonding angle.
Q. Which molecule has a 120 degree bond angle?
Boron trifluoride