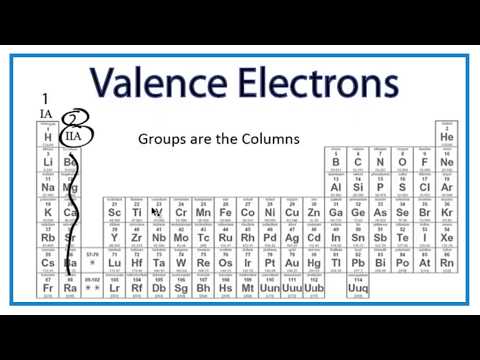
Explanation: Group 2 has two valance electrons. Group 18 would have 8 valance electrons.
Q. Are electrons transferred in a covalent bond?
Covalent Bonds A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions. Shared electrons located in the space between the two nuclei are called bonding electrons.
Table of Contents
- Q. Are electrons transferred in a covalent bond?
- Q. How many electrons are transferred in MGS?
- Q. How many valence electrons are in nitrogen?
- Q. Why nitrogen has 3 Valency?
- Q. Can nitrogen have 6 valence electrons?
- Q. How nitrogen can form 4 bonds?
- Q. Why is nitrogen positive with 4 bonds?
- Q. How many lone pairs are in water?
- Q. Why does h20 have 2 lone pairs?
- Q. How do you calculate lone pairs?
- Q. How many electrons are transferred in ionic bonds?
- Q. Why do ionic bonds steal electrons?
- Q. How does electron transfer work?
- Q. What is the sharing of electrons called?
- Q. What is another name for electrons found in the outer shell?
- Q. Why are there only 8 electrons in the outer shell?
- Q. How many electrons can the first shell hold?
- Q. How do you calculate electrons per shell?
- Q. How many electrons can 4f hold?
- Q. Why can 4f sublevel hold 14 electrons?
- Q. Is 4f possible?
- Q. What is the value of N and L for 4f orbitals?
Q. How many electrons are transferred in MGS?
Mg loses two electrons to have an octet. Oxygen gains two electrons to have an octet. The ionic bond between ions results from the electrostatic attraction of opposite charges.
Q. How many valence electrons are in nitrogen?
5 valence electrons
Q. Why nitrogen has 3 Valency?
The nitrogen atom has 5 electrons in the outermost shell, so it can accept 3 electrons to fulfil the octet structure. Therefore the valency of nitrogen in NH3 is 3.
Q. Can nitrogen have 6 valence electrons?
The total number of valence electrons is 5+6=11. Therefore, no matter how electrons are shared between the nitrogen and oxygen atoms, there is no way for nitrogen to have an octet.
Q. How nitrogen can form 4 bonds?
If you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four covalent bonds. Either with single, double, or triple bonds. It is similar to phosphorus in this regard because they both have five valence electrons (four when they have a positive charge).
Q. Why is nitrogen positive with 4 bonds?
All N-O bonds are polar bonds with more electron density on the oxygen atom. Nitrogen has 5 valence electrons and is in a row with a maximum valence number of 8. It typically forms 3 bonds and has a lone pair (:NH3) or makes 4 bonds with a positive charge (NH4+).
Q. How many lone pairs are in water?
two lone pairs
Q. Why does h20 have 2 lone pairs?
The water molecule is so common that it is wise to just memorize that water is a BENT molecule. The oxygen has 6 valence electrons and thus needs 2 more electrons from 2 hydrogen atoms to complete its octet. This then leaves two lone electron pairs that are not bonded to any other atoms.
Q. How do you calculate lone pairs?
Find the number of lone pairs on the central atom by subtracting the number of valence electrons on bonded atoms (Step 2) from the total number of valence electrons (Step 1). Divide the number of VEs not in bonds (from Step 3) by 2 to find the number of LPs.
Q. How many electrons are transferred in ionic bonds?
The tendency to form species that have eight electrons in the valence shell is called the octet rule. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions.
Q. Why do ionic bonds steal electrons?
An ionic bond is held together by the electrostatic attraction between ions that are near one another. In this type of bond, one atom gives up electrons and becomes a positively charged ion (cation). Another atom dons a ski mask and steals the electrons to become a negatively charged ion (anion).
Q. How does electron transfer work?
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of a redox reaction, wherein the oxidation state of reactant and product changes. Numerous biological processes involve ET reactions.
Q. What is the sharing of electrons called?
When electrons are shared between two atoms, they make a bond called a covalent bond. Chemists frequently use Lewis diagrams to represent covalent bonding in molecular substances.
Q. What is another name for electrons found in the outer shell?
The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons.
Q. Why are there only 8 electrons in the outer shell?
The shells of an atom cannot accommodate more than 8 electrons, even if it has a capacity to accommodate more electrons. This is a very important rule called the Octet rule. According to this rule, atoms gain, loose or share electrons to achieve the stable configuration similar to the nearest noble gas.
Q. How many electrons can the first shell hold?
2
Q. How do you calculate electrons per shell?
Rule 1: The maximum number of electrons present in a particular shell is calculated by the formula 2n2, where “n” represents the shell number. For instance, K shell is the first shell and it can hold up to 2(1)2 = 2 electrons. Similarly, L shell is the second shell and it can hold up to 2(2)2 = 8 electrons.
Q. How many electrons can 4f hold?
Maximum number of orbitals in an energy level (n2)
| Principal Energy Level (n) | sublevels | total electrons |
|---|---|---|
| 1 | 1s | 2 |
| 2 | 2s 2p | 8 |
| 3 | 3s 3p 3d | 18 |
| 4 | 4s 4p 4d 4f | 32 |
Q. Why can 4f sublevel hold 14 electrons?
With reference to quantum numbers, explain why the 4f sublevel can hold a maximum of 14 electrons. Each orbital can have electrons with +1/2 and -1/2 for me. Thus there are two electrons per orbital. Seven orbitals with two electrons per orbital leads to 14 electrons in the 4f sublevel.
Q. Is 4f possible?
with f subshell, l=3 so ml =-3,-2, -1 , 0 ,+1,+2,+3 so there are seven possible values of ml . Remember that 4 is the number of principal quantum number it has nothing to do with the number of the orbitals. So, there will seven 4f orbitals .
Q. What is the value of N and L for 4f orbitals?
Table of Allowed Quantum Numbers
| n | l | Orbital Name |
|---|---|---|
| 4 | 0 | 4s |
| 1 | 4p | |
| 2 | 4d | |
| 3 | 4f |