Q. Is a change of state a chemical or physical change?
Solution: Change in state of matter is a physical change because of the physical condition and appearance changes but not the chemical composition.
Q. Which action is a change in state?
Dissolving is your answer to this question. Becuase when something dissolves it is no longer in the same shape or state cause if something dissovles then goes away into another state.
Table of Contents
- Q. Is a change of state a chemical or physical change?
- Q. Which action is a change in state?
- Q. What happens during change of state?
- Q. What change of state is freezing?
- Q. Which of the following is a change of state?
- Q. Which of the following process is an example of a change of state?
- Q. Why are changes of state reversible?
- Q. Which change of state has the wrong energy?
- Q. What change of state is involved in sweating?
- Q. What ethnicity sweats the most?
- Q. Where do we sweat the most?
- Q. What is the process of sweating?
- Q. What hormone is responsible for sweating?
- Q. What triggers sweating?
- Q. Why is sweating considered an important process?
- Q. Does sweating clean your system?
- Q. Is Sweating Good for you when you sleep?
- Q. Is sweating good for your face?
- Q. What are the disadvantages of sweating?
- Q. Does exercise change your face?
- Q. Is it bad to let sweat dry on your body?
- Q. Is water enough to wash away sweat?
- Q. Why do I feel sticky after sweating?
- Q. Is sweating more good or bad?
- Q. Is sweating everyday good for you?
- Q. Can excessive sweating be a symptom of heart problems?
- Q. What is Ross syndrome?
- Q. What causes a change of state?
- Q. What is meant by change of state in chemistry?
- Q. What are the three changes of state?
- Q. What changes of state require an increase in energy?
- Q. What is needed for states of matter to change?
- Q. Do all substances change their state?
- Q. What are different states of matter?
- Q. What is the common in the three state of matter?
- Q. How are the three states of matter similar and different?
- Q. What are the three states of matter define each of them with two examples?
- Q. Which property is common to all matter?
- Q. What are the two properties that all matter has in common?
- Q. What are two characteristics common to all matter?
- Q. What are the 2 types of properties of matter?
- Q. Where do properties of matter come from?
- Q. How can properties help you distinguish one substance from another?
- Q. What are extensive properties of matter?
- Q. Which one of the following is extensive property?
Q. What happens during change of state?
Changes of state are physical changes. They occur when matter absorbs or loses energy. Processes in which matter changes between liquid and solid states are freezing and melting. Processes in which matter changes between liquid and gaseous states are vaporization, evaporation, and condensation.
Q. What change of state is freezing?
The change from the liquid state to the solid state is called freezing. As the liquid cools, it loses thermal energy. As a result, its particles slow down and come closer together. Attractive forces begin to trap particles, and the crystals of a solid begin to form.
Q. Which of the following is a change of state?
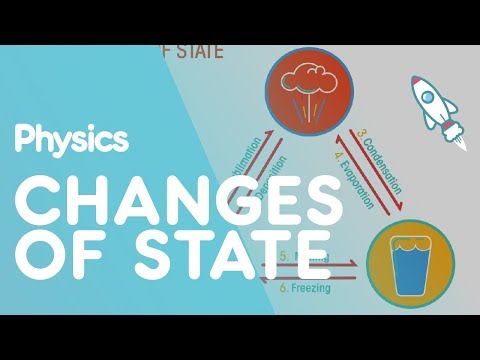
Changes of state are physical changes in matter. They are reversible changes that do not involve changes in matter’s chemical makeup or chemical properties. Common changes of state include melting, freezing, sublimation, deposition, condensation, and vaporization. These changes are shown in Figure below.
Q. Which of the following process is an example of a change of state?
Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Energy is always involved in changes of state. Particles of matter either absorb or lose energy when matter changes from one state to another.
Q. Why are changes of state reversible?
State changes are reversible – ice can be melted and then frozen again. No new elements or compounds are formed. The closeness, arrangement and motion of the particles in a substance change when it changes state. bonds between particles break when a substance melts or evaporates, or sublimes to form a gas from a solid.
Q. Which change of state has the wrong energy?
Answer: Statement-1 : Change in internal energy in the melting process is due to change in internal potential energy. Statement-2 : This is because in melting, distance between molecules increases but temperature remains constant.
Q. What change of state is involved in sweating?
evaporation
Q. What ethnicity sweats the most?
Volume of body sweat increased in both races with rate of walking; volume of hand sweat increased more in Whites than in Blacks. The Mann-Whitney test revealed that volumes of hand sweat were significantly greater for Whites than for Blacks.
Q. Where do we sweat the most?
The most common areas of sweating on the body include:
- armpits.
- face.
- palms of the hands.
- soles of the feet.
Q. What is the process of sweating?
Sweating is the release of liquid from the body’s sweat glands. This liquid contains salt. This process is also called perspiration. Sweating helps your body stay cool.
Q. What hormone is responsible for sweating?
Thermoregulatory stimuli (heat) stimulate the release of acetylcholine (the neurotransmitter) from nerve endings in the eccrine glands and catalyse sweat secretion.
Q. What triggers sweating?
Sweating is your body’s mechanism to cool itself. Your nervous system automatically triggers your sweat glands when your body temperature rises. Sweating also normally occurs, especially on your palms, when you’re nervous. The most common form of hyperhidrosis is called primary focal (essential) hyperhidrosis.
Q. Why is sweating considered an important process?
Sweating, or perspiring, is how the body regulates temperature—sweat keeps us cool and comfortable and prevents the body from overheating in hot environments or during exercise.
Q. Does sweating clean your system?
Sweat is 99% water combined with a small amount of salt, proteins, carbohydrates and urea, says UAMS family medicine physician Dr. Charles Smith. Therefore, sweat is not made up of toxins from your body, and the belief that sweat can cleanse the body is a myth. “You cannot sweat toxins out of the body,” Dr.
Q. Is Sweating Good for you when you sleep?
Night sweats refer to excess sweating during the night. But if your bedroom is unusually hot or you are wearing too many bedclothes, you may sweat during sleep, and this is normal.
Q. Is sweating good for your face?
Sweat is good for the skin. Water hydrates, minerals and salt naturally exfoliate, and urea and uric acid combat dry skin and dermatitis. Sweating purges the skin of bacteria, dirt, oils and impurities. The optimal pH factor for the skin is the same as the pH factor of sweat.
Q. What are the disadvantages of sweating?
Sweating in normal amounts is an essential bodily process. Not sweating enough and sweating too much can both cause problems. The absence of sweat can be dangerous because your risk of overheating increases. Excessive sweating may be more psychologically damaging than physically damaging.
Q. Does exercise change your face?
On top of this, increased circulation from exercise means more oxygen and nutrients are delivered to your skin cells – which will radiate on your face. But you can also enhance elasticity by toning your facial muscles specifically.
Q. Is it bad to let sweat dry on your body?
Sweating is great for your skin unless you leave it there to dry, which may clog the pores. “But make sure you’re cleansing your skin immediately afterward,” says Jodi Dorf, manager and esthetician at Stars Esthetics Spa in Baltimore. Allowing sweat to dry on the skin can clog pores and cause acne.
Q. Is water enough to wash away sweat?
All you absolutely need, bare bones, to stay clean is water. Just water. Water does a fine job of rinsing away dirt without stripping vital oils from your skin. Also, avoid those luxurious long, hot showers.
Q. Why do I feel sticky after sweating?
When it is humid out the atmosphere is already fairly saturated, making it difficult for the sweat from your body to evaporate. Since that sweat can not evaporate, it tends to cling onto the body giving you that overall ‘sticky’ feeling.
Q. Is sweating more good or bad?
Excessive sweating, or hyperhidrosis, can be a warning sign of thyroid problems, diabetes or infection. Excessive sweating is also more common in people who are overweight or out of shape. The good news is that most cases of excessive sweating are harmless.
Q. Is sweating everyday good for you?
From a physiological perspective, sweating is absolutely a good thing. Our body would overheat if we did not sweat. But some of the activities that cause sweating (excessive time in the heat, being nervous or sick) is associated with other problems, such as heat exhaustion, anxiety and illness.
Q. Can excessive sweating be a symptom of heart problems?
Sweating more than usual — especially if you aren’t exercising or being active — could be an early warning sign of heart problems. Pumping blood through clogged arteries takes more effort from your heart, so your body sweats more to try to keep your body temperature down during the extra exertion.
Q. What is Ross syndrome?
Ross syndrome is a rare dysautonomia characterized by a clinical complex of segmental anhidrosis or hypohidrosis, areflexia, and tonic pupils. A very few cases (≃50) have been reported in literature since its original description in 1958.
Q. What causes a change of state?
Changing state Transferring energy to or from a substance can change its state. Heating a substance in the solid state will cause it to melt , which changes it to the liquid state. Continued heating will cause the substance to evaporate or boil, which changes it to the gas state.
Q. What is meant by change of state in chemistry?
A change in state refers to the changing of the arrangement and bonding between particles (and thus shape) of a substance at a given temperature.
Q. What are the three changes of state?
Changes of state are physical changes in matter. They are reversible changes that do not involve changes in matter’s chemical makeup or chemical properties. Common changes of state include melting, freezing, sublimation, deposition, condensation, and vaporization.
Q. What changes of state require an increase in energy?
In order for the molecules to actually separate from each other, more energy must be added. This energy, called heat of fusion or heat of melting, is absorbed by the particles as potential energy as the solid changes to a liquid.
Q. What is needed for states of matter to change?
Matter changes state when energy is added or taken away. Most matter changes because of heat energy. When matter is heated enough, the molecules move faster and with greater energy. If enough heat is added, a solid can become liquid and a liquid can become gas.
Q. Do all substances change their state?
All matter can move from one state to another. It may require extreme temperatures or extreme pressures, but it can be done. Sometimes a substance doesn’t want to change states. To create a solid, you might have to decrease the temperature by a huge amount and then add pressure.
Q. What are different states of matter?
There are three common states of matter:
- Solids – relatively rigid, definite volume and shape. In a solid, the atoms and molecules are attached to each other.
- Liquids – definite volume but able to change shape by flowing. In a liquid, the atoms and molecules are loosely bonded.
- Gases – no definite volume or shape.
Q. What is the common in the three state of matter?
The common things among the three states of matter are: They are made up of small tiny particles. They have a particular mass and can occupy space. The atoms of these three states have force of attractions between them.
Q. How are the three states of matter similar and different?
Gases, liquids and solids are all made up of microscopic particles, but the behaviors of these particles differ in the three phases. gas are well separated with no regular arrangement. liquid are close together with no regular arrangement. solid are tightly packed, usually in a regular pattern.
Q. What are the three states of matter define each of them with two examples?
Three States of Matter
- Matter can exist in one of three main states: solid, liquid, or gas.
- Solid matter is composed of tightly packed particles.
- Liquid matter is made of more loosely packed particles.
- Gaseous matter is composed of particles packed so loosely that it has neither a defined shape nor a defined volume.
Q. Which property is common to all matter?
The two most common properties are intermolecular forces and density. Explanation: Anything that has mass and volume and can occupy space is known as matter. The composition of matter shows that it has small particles.
Q. What are the two properties that all matter has in common?
Matter can be defined or described as anything that takes up space, and it is composed of miniscule particles called atoms. It must display the two properties of mass and volume.
Q. What are two characteristics common to all matter?
All matter has physical and chemical properties. Physical properties are characteristics that scientists can measure without changing the composition of the sample under study, such as mass, color, and volume (the amount of space occupied by a sample).
Q. What are the 2 types of properties of matter?
Key Points
- All properties of matter are either physical or chemical properties and physical properties are either intensive or extensive.
- Extensive properties, such as mass and volume, depend on the amount of matter being measured.
Q. Where do properties of matter come from?
All properties of matter are either extensive or intensive and either physical or chemical. Extensive properties, such as mass and volume, depend on the amount of matter that is being measured. Intensive properties, such as density and color, do not depend on the amount of matter.
Q. How can properties help you distinguish one substance from another?
The characteristics that enable us to distinguish one substance from another are called properties. We can observe some physical properties, such as density and color, without changing the physical state of the matter observed.
Q. What are extensive properties of matter?
An extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount.
Q. Which one of the following is extensive property?
The properties that are independent of the mass or size of the system or which does not depend on the amount of substance are known as intensive properties. Hence volume is an extensive property.