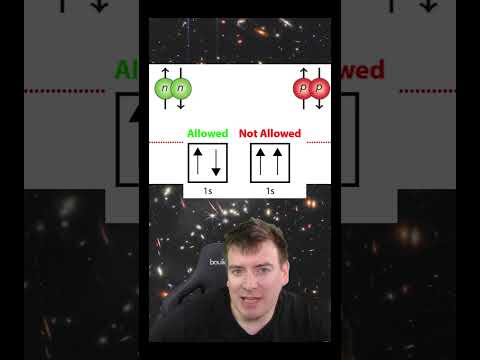
Q. Is a neutron an uncharged particle?
An uncharged elementary particle, with a mass slightly greater than that of the proton, found in the nucleus of every atom heavier than hydrogen.
Q. What is uncharged particle?
Neutron: An uncharged particle found in the nucleus of an atom. A neutron, like a proton, contributes one atomic mass unit to the total atomic weight of an atom. Proton: A positively charged particle found in the nucleus of an atom.
Table of Contents
- Q. Is a neutron an uncharged particle?
- Q. What is uncharged particle?
- Q. How do I find the number of protons electrons and neutrons?
- Q. What is an uncharged atom called?
- Q. Why is an atom neutral?
- Q. Can an atom have no protons?
- Q. What element has most neutrons?
- Q. Can an atom have no electrons?
- Q. What if there were no neutrons?
- Q. Is free neutron a stable particle?
- Q. Can an atom have no neutrons?
- Q. Why do neutrons have no charge?
- Q. What particle has no charge?
- Q. Can we see protons and neutrons?
- Q. Which is bigger neutron or proton?
- Q. Who discovered electron?
- Q. Which subatomic particle is the biggest?
- Q. What is a an electron?
- Q. How old is an electron?
- Q. Why is it called an electron?
- Q. Do electrons have mass?
- Q. Do electrons affect atomic mass?
- Q. Do electrons decay?
- Q. Do electrons have energy?
- Q. Why do electrons repel?
- Q. Why do electrons not lose energy?
- Q. Do electrons physically move?
- Q. Can you stop an electron?
- Q. How do electrons move?
- Q. Why are electrons always moving?
Q. How do I find the number of protons electrons and neutrons?
To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number:
- number of protons = atomic number.
- number of electrons = atomic number.
- number of neutrons = mass number – atomic number.
Q. What is an uncharged atom called?
The Atom’s Charge As long as the number of protons in an atom equals the number of electrons, the atom remains uncharged, or neutral. When an atom gains or loses electrons, it becomes an electrically charged ion.
Q. Why is an atom neutral?
Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. These opposite charges cancel each other out making the atom neutral.
Q. Can an atom have no protons?
Properties. Neutron matter is equivalent to a chemical element with atomic number 0, which is to say that it is equivalent to a species of atoms having no protons in their atomic nuclei.
Q. What element has most neutrons?
helium atoms
Q. Can an atom have no electrons?
Electrically neutral atoms can exist with no electrons. So an atom can’t have no electrons as it, by definition has protons and to be neutral must have electrons. You can have an ion, such as a hydrogen ion (you might call it a proton).
Q. What if there were no neutrons?
Simply, if there are no neutrons, the protons would get separated from the atom by repelling each other and thus the nuclei will be left alone. Therefore there will be no further elements.
Q. Is free neutron a stable particle?
A free neutron is unstable, decaying to a proton, electron and antineutrino with a mean lifetime of just under 15 minutes (879.6±0.8 s). This radioactive decay, known as beta decay, is possible because the mass of the neutron is slightly greater than the proton. The free proton is stable.
Q. Can an atom have no neutrons?
There is only one stable atom that does not have neutrons. It is an isotope of the element hydrogen called protium. Protium, which contains a single proton and a single electron, is the simplest atom. All other stable atoms contain some number of neutrons.
Q. Why do neutrons have no charge?
Like all hadrons, neutrons are made of quarks. A neutron is made of two down quarks and one up quark. One up quark has a charge of +2/3, and the two down quarks each have a charge of -1/3. The fact that these charges cancel out is why neutrons have a neutral (0) charge.
Q. What particle has no charge?
Neutron
Q. Can we see protons and neutrons?
We can never see the subatomic particles directly, but can only infer from observation of such indirect effects like tracks. If there are many of them and they are emitting some radiation, and also if we shine some radiation on then and receive back the response this will also constitute a kind of seeing.
Q. Which is bigger neutron or proton?
The mass of a neutron is slightly greater than the mass of a proton, which is 1 atomic mass unit (amu). (An atomic mass unit equals about 1.kilograms.) A neutron also has about the same diameter as a proton, or 1.7×10−15 meters.
Q. Who discovered electron?
Thomson
Q. Which subatomic particle is the biggest?
neutron
Q. What is a an electron?
An electron is a negatively charged subatomic particle. It can be either free (not attached to any atom), or bound to the nucleus of an atom. Electrons in atoms exist in spherical shells of various radii, representing energy levels. The charge on a single electron is considered as the unit electrical charge.
Q. How old is an electron?
The best measurement yet of the lifetime of the electron suggests that a particle present today will probably still be around in 66,000 yottayears (6.6 × 1028 yr), which is about five-quintillion times the current age of the universe.
Q. Why is it called an electron?
he word “electron,” coined by G. Johnstone Stoney in 1891, had been used to denote the unit of charge found in experiments that passed electric current through chemicals. In this sense the term was used by Joseph Larmor, J.J. Thomson’s Cambridge classmate.
Q. Do electrons have mass?
Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton.
Q. Do electrons affect atomic mass?
Electrons are much smaller in mass than protons, weighing only 9.11 × 10-28 grams, or about 1/1800 of an atomic mass unit. Therefore, they do not contribute much to an element’s overall atomic mass. Protons, neutrons, and electrons: Both protons and neutrons have a mass of 1 amu and are found in the nucleus.
Q. Do electrons decay?
And since the electron is the lightest particle that has electric charge, there is nothing that it can decay to; only neutrinos, photons, gluons and gravitons are lighter, but they are all electrically neutral, so any combination of them would have zero electric charge.
Q. Do electrons have energy?
The ground state of an electron, the energy level it normally occupies, is the state of lowest energy for that electron. There is also a maximum energy that each electron can have and still be part of its atom.
Q. Why do electrons repel?
An electron has the opposite charge properties to a positive charge, and a free electron will move in the direction that opposes the force lines. So an electron will move away from another negative charge, and towards a positive charge. The force experienced by a charge is F=E⋅q. So the electrons do repel each other.
Q. Why do electrons not lose energy?
1) If an electron is in the electric field of a nucleus, the electron can occupy only certain energy levels. When it is sitting on one of these energy levels, it does not radiate, it does not loose energy.
Q. Do electrons physically move?
Electrons do physically move when a voltage is applied – extremely slowly. This works out to 8.4 cm/hour.
Q. Can you stop an electron?
No, it’s not possible to stop an electron. because of the simple fact, it has to obey the Heisenberg uncertainty relation with respect to place and momentum. In the extreme case (theoretically) we can measure the electron’s momentum with absolute certainty.
Q. How do electrons move?
The electrons move from negatively charged parts to positively charged ones. The negatively charged pieces of any circuit have extra electrons, while the positively charged pieces want more electrons. The electrons then jump from one area to another. When the electrons move, the current can flow through the system.
Q. Why are electrons always moving?
Because opposite electric charges attract each other, negative electrons are attracted to the positive nucleus. This force of attraction keeps electrons constantly moving through the otherwise empty space around the nucleus.