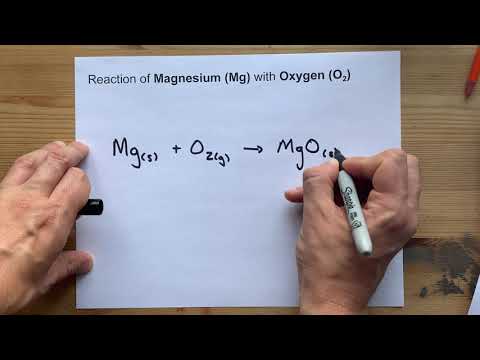
For example, the synthesis of magnesium oxide, MgO , is a redox reaction in which oxygen gas oxidizes magnesium metal, while being reduced in the process. Each magnesium atom loses two electrons, so two magnesium atoms will lose a total of four electrons.
Q. What type of reaction is the reaction of magnesium with oxygen?
Oxygen and magnesium combine in a chemical reaction to form this compound. After it burns, it forms a white powder of the magnesium oxide. Magnesium gives up two electrons to oxygen atoms to form this powdery product. This is an exothermic reaction.
Table of Contents
- Q. What type of reaction is the reaction of magnesium with oxygen?
- Q. What happens when a magnesium reacts with oxygen Class 7?
- Q. How do you stop magnesium from reacting with nitrogen?
- Q. What is the evidence that the magnesium is reacting?
- Q. Does magnesium react with oxygen at room temperature?
- Q. What does magnesium look like?
- Q. Can I take magnesium with a multivitamin?
- Q. Can you take magnesium with vitamin D?
Q. What happens when a magnesium reacts with oxygen Class 7?
When magnesium ribbon burns in air, then the magnesium metal combines with the oxygen (of air) to form a new substance called magnesium oxide.
Q. How do you stop magnesium from reacting with nitrogen?
By immersing it in an atmosphere of CO2, you can eliminate the reaction with nitrogen so that no Mg3N2 is formed (or at least very minimal amounts from the transfer). What you get instead is a mixture of MgO (white powder) and black carbon ash.
Q. What is the evidence that the magnesium is reacting?
Evidence: When magnesium is placed in a Bunsen burner flame in air it burns with a bright, white flame. The product is a white powdery solid. Explanation: At high temperatures the magnesium atoms in the metal combine with the oxygen atoms in the air. A chemical reaction produces magnesium oxide.
Q. Does magnesium react with oxygen at room temperature?
Magnesium metal, and its alloys, are explosive hazards and must be handled with extreme caution. When magnesium metal burns it reacts with oxygen found in the air to form Magnesium Oxide, which is a compound. Intense heating is required to make magnesium burn in oxygen . HOPE IT HELPS YOU!!!!
Q. What does magnesium look like?
A silvery-white metal that ignites easily in air and burns with a bright light. Magnesium is one-third less dense than aluminium.
Q. Can I take magnesium with a multivitamin?
If You Take Mineral Supplements Large doses of minerals can compete with each other to be absorbed. Don’t use calcium, zinc, or magnesium supplements at the same time.
Q. Can you take magnesium with vitamin D?
If you’re looking to get more vitamin D in your diet, take it with a side of magnesium. That mineral appears to help regulate levels of vitamin D, which in turn manages the levels of other minerals such as calcium and phosphorus.