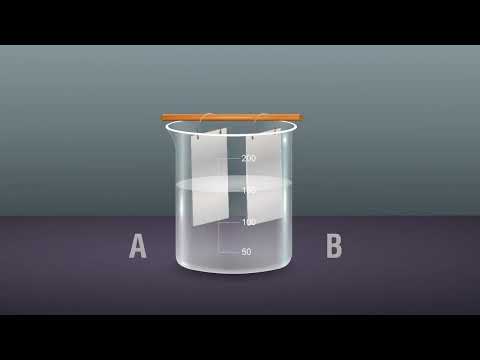
Q. Is sodium chloride conductive in water?
Common table salt (NaCl) is an electrolyte, and when this is dissolved in water to form salt water, it becomes sodium ions (Na+) and chloride ions (Cl-), each of which is a corpuscle that conducts electricity.
Q. Is sodium chloride conductive without water?
Pure water is not very conductive, and only a tiny bit of current can move through the water. When salt or sodium chloride (NaCl) is dissolved in it, however, the salt molecules split into two pieces, a sodium ion and a chlorine ion. The sodium ion is missing an electron, which gives it a positive charge.
Table of Contents
- Q. Is sodium chloride conductive in water?
- Q. Is sodium chloride conductive without water?
- Q. Why is sodium chloride conductive in water?
- Q. Does sodium chloride have a high conductivity?
- Q. Do different salts have different conductivity?
- Q. Is Salt ionic conductor?
- Q. Is Honey conductor of electricity?
- Q. Is 99% isopropyl alcohol conductive?
- Q. Is beer more conductive than water?
- Q. Why is pure water an insulator?
Q. Why is sodium chloride conductive in water?
When the sodium chloride dissolves in water, the sodium atoms and chlorine atoms separate under the influence of the water molecules. They’re free to move around in the water as positively and negatively charged ions. This separation of charge allows the solution to conduct electricity.
Q. Does sodium chloride have a high conductivity?
In the frequency range above 50 Hz, the electrical impedance of the NaCl solution tends to be constant; this is because the NaCl solution is an ionic solution having greater conductivity properties.
Q. Do different salts have different conductivity?
The conductivity is determined by the number of charge carriers, how fast they move, and how much charge each one carries. Hence, for most aqueous solutions, the higher the concentration of dissolved salts, which will lead to more ions, the higher the conductivity.
Q. Is Salt ionic conductor?
Explanation: Salts are ionic compounds. Ionic compounds cannot conduct electricity when solid because, although they are entirely composed of charged particles called ions, these ions are not free to move: a factor vital to conduction of electricity. This static inertia is not maintained when the compound is melted.
Q. Is Honey conductor of electricity?
Honey is a solution of sugars. So, it does not conduct because it does not have ions or charged particles.
Q. Is 99% isopropyl alcohol conductive?
Alcohol (isopropyl alcohol, ethanol and isopropanol) is a polar solvent (very conductive) and is potentially corrosive (contains water). Not recommended as a carrier solvent. Alcohol is not recommended in concentrations greater than 3% of the total formulation.
Q. Is beer more conductive than water?
When they tested conductivity, the results were surprising. Whiskey did well, beer did better, and red wine was streaks ahead with a whopping 63% of the material showing superconductive properties. For some reason commercial drinks created better superconductors than pure ethanol and water.
Q. Why is pure water an insulator?
Since pure water does not contain any impurities Ca, Mg, Na and other salts which behave as the charge carriers, there are no ions found, and there is the absence of electrons. Hence pure water does not conduct electricity and is an insulator.